Chapter 124 Disorders of Phagocyte Function
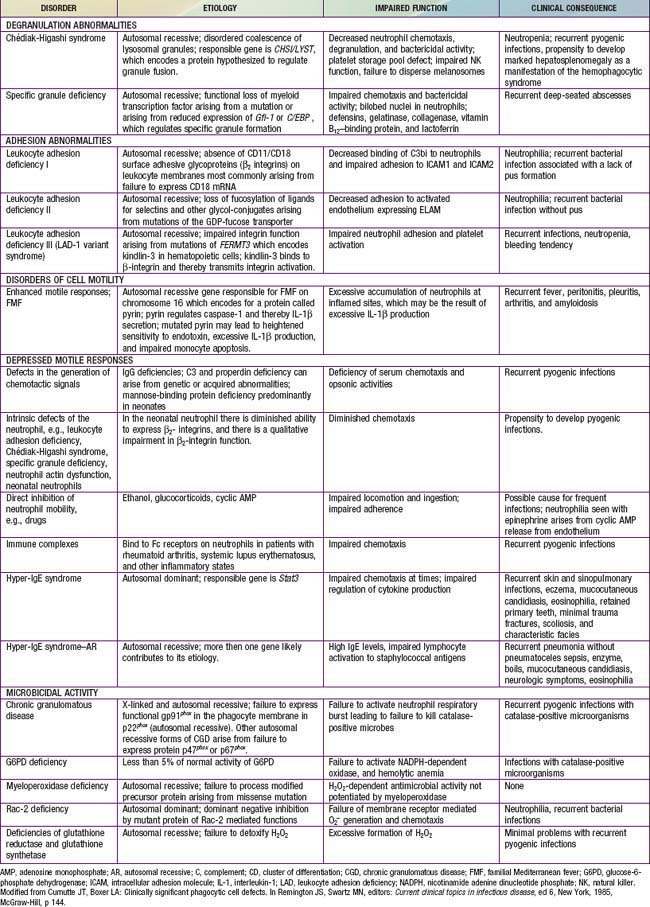
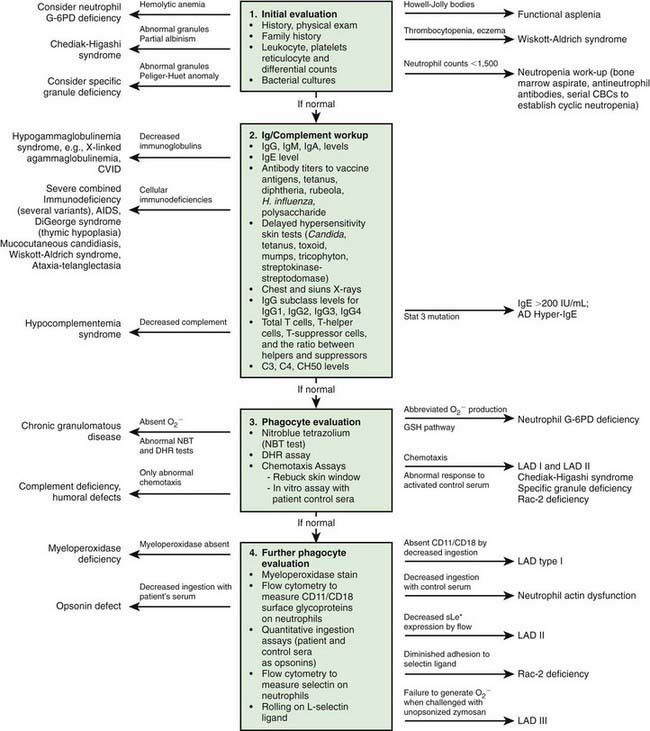
Immunologic evaluation of patients with suspected immunodeficiency (Chapter 116) should focus on disorders of phagocyte function (Table 124-1) in patients with recurrent or unusual bacterial infections (Fig. 124-1).
Chemotaxis, the direct migration of cells into sites of infection, involves a complex series of events (Chapter 121). Studies of defective in vitro chemotaxis of neutrophils obtained from children having various clinical conditions have not established whether frequent infections arise from a primary chemotactic abnormality or occur as secondary medical complications of the underlying disorder. For example, variable but at times severe abnormalities in neutrophil motility accompany the hyper-IgE syndrome, which is characterized by markedly elevated levels of IgE, chronic dermatitis, and recurrent sinopulmonary infections, as well as coarse facial features, retention of primary teeth, and a propensity for recurrent bone fractures (Chapter 123).
Leukocyte Adhesion Deficiency
Genetics and Pathogenesis
Mutations in the CD18 gene either impair gene expression or affect the structure of the synthesized CD18 peptide, leading to functionally abnormal CD11/CD18. Some mutations of CD11/CD18 allow a low level of assembly and activity of integrin molecules. These children retain some neutrophil integrin adhesion function and have a moderate phenotype. Failure of neutrophils to bear the β2-integrins leads to inability to migrate to sites of inflammation outside the blood vessel lumen because of their inability to adhere firmly to surfaces and undergo transendothelial migration. Failure of the CD11/CD18–deficient neutrophils to undergo transendothelial migration occurs because the β2-integrins bind to intercellular adhesion molecules 1 (ICAM-1) and 2 (ICAM-2) expressed on inflamed endothelial cells (Chapter 121). Neutrophils that do arrive at inflammatory sites by CD11/CD18–independent processes fail to recognize microorganisms opsonized with complement fragment iC3b, an important stable opsonin formed by the cleavage of C3b. Hence, other neutrophil functions such as degranulation and oxidative metabolism normally triggered by iC3b binding are also markedly compromised in LAD-1 neutrophils, resulting in impaired phagocytic function and high risk for serious and recurrent bacterial infections.
Clinical Manifestations
The pathogens infecting patients with LAD-1 are similar to those affecting patients with severe neutropenia (Chapter 125) and include Staphylococcus aureus and enteric gram-negative organisms such as Escherichia coli. These patients are also susceptible to opportunistic infection by fungi such as Candida and Aspergillus. Typical signs of inflammation such as swelling, erythema, and warmth may be absent. Pus does not form, and few neutrophils are identified microscopically in biopsy specimens of infected tissues. Despite the paucity of neutrophils within the affected tissue, the circulating neutrophil count during infection typically exceeds 30,000/µL and can surpass 100,000/µL. During intervals between infections, the peripheral blood neutrophil count may chronically exceed 12,000/µL. LAD-1 genotypes producing moderate amounts of functional integrins at the surface of the neutrophil significantly reduce the severity and frequency of infections compared to children with the severe form.