69 Disorders of Calcium and Bone Metabolism
Regulation of Serum Calcium and Phosphorus
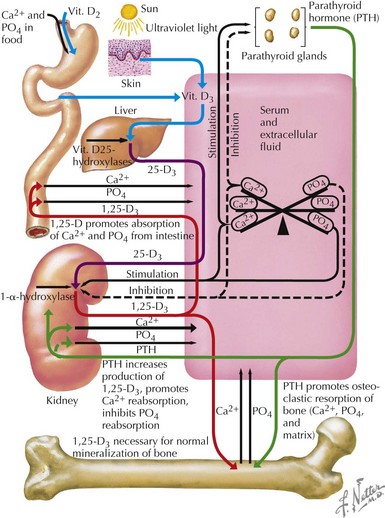
Most (99%) of the body’s calcium exists as hydroxyapatite in bone, with the remaining 1% present in extracellular fluids. Serum calcium exists in three fractions: 50% to 55% is free (ionized) calcium; about 10% is complexed with low-molecular-weight anions; and 35% to 40% is bound to proteins, mainly albumin and, to a lesser extent, globulins. The calciotropic hormones calcitriol (the fully active form of vitamin D) and parathyroid hormone (PTH) act on their target organs, kidney, intestines, and bone to regulate mineral homeostasis (Figure 69-1). Phosphatonins such as FGF23 also play important regulatory roles in mineral metabolism and complement the actions of other calciotropic hormones; phosphatonins decrease renal phosphorus reabsorption while reducing synthesis of calcitriol and secretion of PTH.
Dynamics of Bone Homeostasis
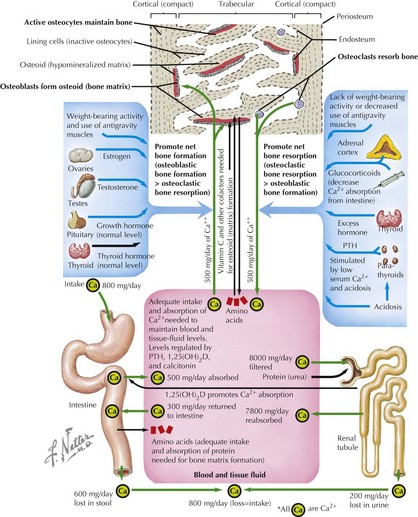
Bone modeling and remodeling are regulated by a variety of factors such as biomechanical loading, hormonal balance, acid–base status, and drug exposures (Figure 69-2). Bone adapts its strength in response to the magnitude and direction of the forces to which it is subjected. Mechanical forces on the skeleton arise primarily from muscle contraction. This capacity of bone to respond to mechanical loading with increased bone size and strength is greatest during growth, especially during puberty and adolescence. Increased production of estrogen and testosterone in addition to increased pulsatile secretion of growth hormone are the hormonal hallmarks of puberty. These events act in an anabolic manner on bone to promote net bone formation.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree