Surfactant Deficiency
Inadequate surfactant secretion that leads to respiratory distress syndrome becomes less frequent with increasing gestational age. That said, even with a low incidence in term infants, RDS from surfactant deficiency is not rare (Berthelot-Ricou, 2012). Male gender and white race are independent risk factors (Anadkat, 2012). Also, mutations of genes that encode for surfactant protein synthesis may augment the deficiency (Garmany, 2008; Wambach, 2012). Regardless of etiology, when surfactant secretion is diminished, the pulmonary pathophysiology, clinical course, and management are similar to that for preterm infants. Treatment includes mechanical ventilation and replacement of surfactant by insufflation (Chap. 34, p. 654). There is no evidence that antenatal maternal corticosteroid treatment will enhance surfactant synthesis in late preterm fetuses (Gyamfi-Bannerman, 2012). The prognosis in term newborns largely depends on the cause, severity, and response to treatment.
 Meconium Aspiration Syndrome
Meconium Aspiration Syndrome
The physiology of meconium passage and amnionic fluid contamination is considered in detail in Chapter 24 (p. 493). In some instances, inhalation of meconium-stained fluid at or near delivery causes acute airway obstruction, chemical pneumonitis, surfactant dysfunction or inactivation, and pulmonary hypertension (Swarnam, 2012). If severe, hypoxemia may lead to neonatal death or long-term neurological sequelae in survivors.
Given the high frequency—10 to 20 percent—of meconium-stained amnionic fluid in laboring women at term, it is reasonable to assume that meconium aspiration must be relatively common. Fortunately, severe aspiration leading to overt respiratory failure is much less frequent. And although the exact incidence of meconium aspiration is not known, Singh and associates (2009) reported it to be 1.8 percent for all deliveries. In a French study of nearly 133,000 term newborns, the prevalence of severe aspiration syndrome was 0.07 percent, and this increased progressively from 37 to 43 weeks’ gestation (Fischer, 2012). Mortality rates depend on severity. In an earlier report from Parkland Hospital, for all patients, meconium aspiration caused 1 death per 1000 live births (Nathan, 1994).
Fetal morbidity is more often associated with thicker meconium content. It is presumed that in most cases, amnionic fluid is ample to dilute the meconium to permit prompt clearance by normal fetal physiological mechanisms. Meconium aspiration syndrome occasionally develops with light staining. Many newborns are affected after a normal labor and uncomplicated delivery. However, some associated obstetrical factors include postterm pregnancy and fetal-growth restriction. These fetuses are at highest risk because there frequently is diminished amnionic fluid and labor with cord compression or uteroplacental insufficiency. These may increase the likelihood of meconium passage that is thick and undiluted (Leveno, 1984).
Prevention
It was once widely held that aspiration was stimulated by fetal hypoxic episodes, and fetal heart rate tracing abnormalities were used to identify fetuses at greatest risk during labor. Unfortunately, this was found to be an unreliable predictor (Dooley, 1985). Preliminary studies reported that the incidence and severity of the syndrome could be mitigated by oropharyngeal suctioning after delivery of the fetal head, but before the chest. For a while, this was standard care, but it was abandoned when shown that strict adherence to this protocol did not reduce syndrome incidence or severity (Davis, 1985; Wiswell, 1990). At the same time, reports described that pulmonary hypertension caused by aspirated meconium was characterized by abnormal arterial muscularization beginning well before birth. These findings led some to conclude that only chronically asphyxiated fetuses developed meconium aspiration syndrome (Katz, 1992). Proof of this never materialized, and Richey (1995) and Bloom (1996) and their colleagues found no correlation between meconium aspiration and markers of acute asphyxia—for example, umbilical artery acidosis. Conversely, others have reported that thick meconium is an independent risk factor for neonatal acidosis (Maisonneuve, 2011).
Conflicted findings regarding suctioning stimulated performance of an 11-center randomized trial designed to compare suctioning with no suctioning (Vain, 2004). There was an identical 4-percent incidence of meconium aspiration syndrome in both groups. Subsequently, a committee that represented the American Heart Association and American Academy of Pediatrics (Perlman, 2010), as well as the American College of Obstetricians and Gynecologists (2013b), has recommended against routine intrapartum oro- and nasopharyngeal suctioning at delivery. Instead, for depressed newborns, management includes intubation and suctioning to remove meconium and other aspirated material from beneath the glottis (Chap. 32, p. 626).
Intrapartum amnioinfusion has been used successfully in laboring women with decreased amnionic fluid volume and frequent variable fetal heart rate decelerations (Chap. 24, p. 494). It has also been studied as a preventive measure in labors complicated by meconium staining (Spong, 1994). Although amnioinfusion apparently posed little or no increased risk, it was found to be of no benefit because fetuses usually inhaled meconium before labor (Bryne, 1987; Wenstrom, 1995). To further settle this issue, a trial was conducted with almost 2000 women at 36 weeks’ gestation or later and in whom labor was complicated by thick meconium (Fraser, 2005). The perinatal death rate with and without amnioinfusion was 0.05 percent in both groups. Rates of moderate or severe meconium aspiration were also not significantly different—4.4 percent with and 3.1 percent without amnioinfusion. Finally, cesarean delivery rates were similar—32 versus 29 percent, respectively. Currently, the American College of Obstetrics and Gynecologists (2012c) does not recommend amnioinfusion to reduce meconium aspiration syndrome.
Treatment
Ventilatory support is given as needed. Because some aspects of meconium aspiration syndrome are caused by surfactant deficiency, replacement therapy has been given in some studies. Extracorporeal membrane oxygenation—ECMO—therapy is reserved for neonates who remain poorly oxygenated despite maximal ventilatory assistance. In their Cochrane review of randomized trials, El Shahed and colleagues (2007) found that surfactant replacement significantly decreased need for extracorporeal membrane oxygenation. Although they found that surfactant administration did not lower the mortality rate, a subsequent trial suggested that it was reduced (Dargaville, 2011). The proportion that requires ECMO treatment varies. In the report by Singh and coworkers (2009), 1.4 percent of 7518 term newborns with the syndrome required such treatment, and these had a 5-percent mortality rate. Ramachandrappa and colleagues (2011) reported a higher mortality rate in late-preterm infants compared with term infants. Finally, pulmonary lavage with surfactant is being evaluated (Choi, 2012).
NEONATAL ENCEPHALOPATHY AND CEREBRAL PALSY
Few events evoke more fear and apprehension in parents and obstetricians than the specter of “brain damage,” which immediately conjures visions of disabling cerebral palsy and hopeless intellectual disability. Although most brain disorders or injuries are less horrific, history has helped to perpetuate the more dismal outlook. In the first edition of this textbook, J. Whitridge Williams (1903) limited discussions of brain injury to that sustained from birth trauma. When later editions introduced the concept that asphyxia neonatorum was another cause of cerebral palsy, this too was linked to traumatic birth. Even as brain damage caused by traumatic delivery became uncommon during the ensuing decades, the belief—albeit erroneous—was that intrapartum events caused most neurological disability. This was a major reason for the escalating cesarean delivery rate beginning in the 1970s. Unfortunately, because in most cases the genesis of cerebral palsy occurred long before labor, this did little to mitigate risks for cerebral palsy (O’Callaghan, 2013).
These realizations stimulated scientific investigations to determine the etiopathogenesis of fetal brain disorders to include those leading to cerebral palsy. Seminal observations include those of Nelson and Ellenberg (1984, 1985, 1986a), discussed subsequently. These investigators are appropriately credited with proving that these neurological disorders are due to complex multifactorial processes caused by a combination of genetic, physiological, environmental, and obstetrical factors. Importantly, these studies showed that few neurological disorders were associated with peripartum events. Continuing international interest was garnered to codify the potential role of intrapartum events. In 2000, President Frank Miller of the American College of Obstetricians and Gynecologists appointed a task force to study the vicissitudes of neonatal encephalopathy and cerebral palsy. From this, the 2003 Task Force findings were promulgated by the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics (2003). The multispecialty coalition reviewed contemporaneous data and provided criteria to define various neonatal brain disorders.
In 2010, 10 years later, President Richard Waldman of the American College of Obstetricians and Gynecologists appointed a second task force to update these findings. The 2014 Task Force has now published its findings (American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, 2014). The 2014 Task Force findings are more circumspect in contrast to the earlier ones. Specifically, more limitations are cited in identifying cause(s) of peripartum hypoxic-ischemic encephalopathy compared with other etiologies of neonatal encephalopathy. The 2014 Task Force recommends multidimensional assessment of each affected infant. They add the caveat that no one strategy is infallible, and thus, no single strategy will achieve 100-percent certainty in attributing a cause to neonatal encephalopathy.
 Neonatal Encephalopathy
Neonatal Encephalopathy
The 2014 Task Force defined neonatal encephalopathy as a syndrome of neurological dysfunction identified in the earliest days of life in neonates born at ≥ 35 weeks’ gestation. It is manifested by subnormal levels of consciousness or seizures and often accompanied by difficulty with initiating and maintaining respiration and by depressed tone and reflexes. The incidence of encephalopathy has been cited to be 0.27 to 1.1 per 1000 term liveborn neonates, and it is much more frequent in preterm infants (Ensing, 2013; Plevani, 2013; Takenouchi, 2012; Wu, 2011). Although the 2014 Task Force concluded that there are many causes of encephalopathy and cerebral palsy, it focused on those termed hypoxic-ischemic encephalopathy (HIE) and thought to be incurred intrapartum. To identify affected infants, a thorough evaluation is necessary, including maternal history, obstetrical antecedents, intrapartum factors, placental pathology, and newborn course, along with laboratory and neuroimaging findings.
There are three clinically defined levels. Mild encephalopathy is characterized by hyperalertness, irritability, jitteriness, and hypertonia and hypotonia. Moderate encephalopathy is manifest by lethargy, severe hypertonia, and occasional seizures. Severe encephalopathy is manifest by coma, multiple seizures, and recurrent apnea.
The 2014 Task Force also concluded that of the several forms of cerebral palsy, only the spastic quadriplegic type can result from acute peripartum ischemia. Other forms—hemiparetic or hemiplegic cerebral palsy, spastic diplegia, and ataxia—are unlikely to result from an intrapartum event. Purely dyskinetic or ataxic cerebral palsy, especially when accompanied by a learning disorder, usually has a genetic origin (Nelson, 1998).
Criteria for Hypoxic-Ischemic Encephalopathy
The 2014 Task Force radically revised its 2003 criteria used to define an acute peripartum event that is consistent with a hypoxic-ischemic episode and neonatal encephalopathy. These are outlined in Table 33-1 and are considered with the following caveats.
TABLE 33-1. Findings Consistent with an Acute Peripartum or Intrapartum Event Leading to Hypoxic-Ischemic Encephalopathy
Neonatal Findings
Apgar score: < 5 at 5 and 10 minutes
Umbilical arterial acidemia: pH < 7.0 and/or base deficit ≥ 12 mmol/L
Neuroimaging evidence of acute brain injury: MRI or MRS consistent with HIE
Multisystem involvement consistent with HIE
Type and Timing of Contributing Factors
Sentinel hypoxic or ischemic event occurring immediately before or during delivery
Fetal heart rate monitor patterns consistent with an acute peripartum or intrapartum event
Apgar Scores. Low 5- and 10-minute Apgar scores are associated with increased risks for neurological impairment. There are many causes of low Apgar scores, and most of these infants will not develop cerebral palsy. If the 5-minute Apgar is ≥ 7, it is unlikely that peripartum HIE caused cerebral palsy.
Acid-Base Studies. Low pH and base deficit levels increase the likelihood that neonatal encephalopathy was caused by HIE. Decreasing levels form a continuum of increasing risk, but most acidemic neonates will be neurologically normal (Wayock, 2013). A cord artery pH > 7.2 is very unlikely to be associated with HIE.
Neuroimaging Studies. Magnetic resonance (MR) imaging or spectroscopy (MRS) is the best modality with which to visualize findings consistent with HIE. The 2014 Task Force concludes that cranial sonography and computed tomography (CT) lack sensitivity in the term newborn. Normal MR imaging/MRS findings after the first 24 hours of life, however, effectively exclude a hypoxic-ischemic cause of encephalopathy. MR imaging between 24 and 96 hours may be more sensitive for the timing of peripartum cerebral injury, and MR imaging at 7 to 21 days following birth is the best technique to delineate the full extent of cerebral injury.
Multisystem Involvement. Neonatal manifestations of multisystem injury are consistent with HIE. These include renal, gastrointestinal, hepatic, or cardiac injury; hematological abnormalities; or combinations of these. The severity of neurological injury does not necessarily correlate with injuries to these other systems.
Contributing Factors
The 2014 Task Force also found that certain contributing factors may be consistent with an acute peripartum event.
Sentinel Event. Adverse obstetrical events that may lead to catastrophic clinical outcomes are designated sentinel events. Examples given by the 2014 Task Force include ruptured uterus, severe placental abruption, cord prolapse, and amnionic-fluid embolism. Martinez-Biarge and associates (2012) studied almost 58,000 deliveries and identified 192 cases with one of these sentinel events. Of these 192 infants, 6 percent died intrapartum or in the early neonatal period, and 10 percent developed neonatal encephalopathy. In a study of 307 cases of cord prolapse, the fetal death rate was 6.8 percent (Hartigan, 2013). Besides these sentinel events, other risk factors for neonatal acidosis include prior cesarean delivery, maternal age ≥ 35 years, thick meconium, chorioamnionitis, and general anesthesia (Johnson, 2014; Maisonneuve, 2011).
Fetal Heart Rate Patterns. The 2014 Task Force emphasized the importance of differentiating an abnormal fetal heart rate (FHR) tracing on presentation versus one that develops subsequently. A category 1 or 2 FHR tracing associated with Apgar scores ≥ 7 at 5 minutes, normal cord gases (±1 SD), or both, is not consistent with an acute HIE event (Anderson, 2013). An FHR pattern at the time of presentation with persistently minimal or absent variability and lacking accelerations, with duration ≥ 60 minutes, and even without decelerations is suggestive of an already compromised fetus. The Task Force further recommended that if fetal well-being cannot be established with these findings present, the woman should be evaluated for the method and timing of delivery.
Application of 2003 Task Force Criteria
It seemed likely that strict adherence to all of the criteria set forth by the 2003 Task Force would exclude some infants who go on to develop cerebral palsy as a result of neonatal encephalopathy (Strijbis, 2006). To test these criteria, Phelan and coworkers (2011) retrospectively applied the 2003 Task Force criteria to 39 term infants with permanent central nervous system impairment. They found that the Task Force criteria and these 39 cases correlated in varying degrees. Of the essential criteria, pH < 7.0 and base deficit ≥ 12 mmol/L showed a 97- and 100-percent correlation, respectively; moderate to severe encephalopathy—97 percent correlation; spastic quadriplegia or dyskinetic form—92 percent; and no other identifiable cause—100 percent. Of the nonspecific criteria, a sentinel hypoxic event had an 80-percent correlation; fetal heart rate requirements—100 percent; Apgar score ≤ 3 beyond 5 minutes—29 percent; and multisystem involvement—100 percent. As discussed subsequently, a sentinel event alone had only a 10-percent predictive value for newborn encephalopathy (Martinez-Biarge, 2012).
Prevention
Most prophylactic measures for neonatal encephalopathy have been evaluated in preterm infants (Chap. 42, p. 854). One of these—hypothermia—may prevent death and mitigate moderate to severe neurological disability in term infants (Nelson, 2014; Pfister, 2010; Shankaran, 2005, 2012). MR imaging studies have demonstrated a slowing of diffusional abnormalities and fewer infarctions with hypothermia (Bednarek, 2012; Shankaran, 2012). Most randomized trials have shown improved outcomes with hypothermia in infants born at 36 weeks or older (Azzopardi, 2009; Guillet, 2012; Jacobs, 2011). In a metaanalysis of more than 1200 newborns, Tagin and colleagues (2012) concluded that hypothermia improves survival rates and neurodevelopment. More recently, clinical trials to evaluate concomitant erythropoietin therapy for neuroprophylaxis are being conducted (Wu, 2012). Preliminary data from a Dutch multicenter trial of maternal allopurinol therapy indicate some mitigation of cerebral damage from hypoxia and ischemia (Kaandorp, 2013).
 Cerebral Palsy
Cerebral Palsy
This term refers to a group of nonprogressive disorders of movement or posture caused by abnormal development or damage to brain centers for motor control. Cerebral palsy is further classified by the type of neurological dysfunction—spastic, dyskinetic, or ataxic—as well as the number and distribution of limbs involved—quadriplegia, diplegia, hemiplegia, or monoplegia. Together, the major types are spastic quadriplegia—the most common—which has a strong association with mental retardation and seizure disorders; diplegia, which is common in preterm or low-birthweight infants; hemiplegia; choreoathetoid types; and mixed varieties. Although epilepsy and mental retardation frequently accompany cerebral palsy, these two disorders seldom are associated with perinatal asphyxia in the absence of cerebral palsy.
Incidence and Epidemiological Correlates
According to the Centers for Disease Control and Prevention (2011), the prevalence of cerebral palsy is variably reported in the United States. In one multisite surveillance program in 2006, the average prevalence was 2.9 per 1000 8-year-old children. It is crucial to emphasize that this rate is derived from all children—including preterm infants. Because of the remarkably increased survival rates of the latter, the overall rate of cerebral palsy and other developmental disabilities reported in the 1950s has remained essentially unchanged (Boyle, 2011). Long-term follow-up studies of more than 900,000 Norwegian nonanomalous term infants cite an incidence of 1 per 1000 (Moster, 2008). By contrast, the incidence was 91 per 1000 for infants born at 23 to 27 weeks. In absolute numbers, term infants comprise half of cerebral palsy cases because there are proportionately far fewer preterm births. It is again emphasized that most studies have not made distinctions between term and preterm infants.
As noted earlier, Nelson and Ellenberg (1984, 1985, 1986a) made many foundational observations concerning cerebral palsy. Their initial studies emanated from data from the Collaborative Perinatal Project. This included children from almost 54,000 pregnancies who were followed until age 7. They found that the most frequently associated risk factors for cerebral palsy were: (1) evidence of genetic abnormalities such as maternal mental retardation or fetal congenital malformations; (2) birthweight < 2000 g; (3) birth before 32 weeks; and (4) perinatal infection. They also found that obstetrical complications were not strongly predictive, and only a fifth of affected children had markers of perinatal asphyxia. For the first time, there was solid evidence that the cause of most cases of cerebral palsy was unknown, and importantly, only a small proportion was caused by neonatal hypoxic-ischemic encephalopathy. Equally importantly, there was no foreseeable single intervention that would likely prevent a large proportion of cases.
Numerous studies have since confirmed many of these findings and identified an imposing list of other risk factors that are shown in Table 33-2. As expected, preterm birth continues to be the single most important risk factor (Goepfert, 1996; Thorngren-Jerneck, 2006). Small-for-gestational-age infants are also at increased risk. Stoknes and associates (2012) showed that in more than 90 percent of growth-restricted infants, cerebral palsy was due to antepartum factors. A myriad other placental and neonatal risk factors has been correlated with neurodevelopmental abnormalities (Ahlin, 2013; Avagliano, 2010; Blair, 2011; Redline, 2005, 2008). Some placental factors are discussed further in Chapter 6, and in just one example, there is a substantively increased risk with chorioamnionitis (Gilbert, 2010; Shatrov, 2010). An example of a neonatal cause is arterial ischemic stroke, which may be associated with inherited fetal thrombophilias (Gibson, 2005; Harteman, 2013; Kirton, 2011). Also, neonates with isolated congenital heart lesions have an increased risk for microcephaly, possibly due to chronic fetal hypoxemia (Barbu, 2009). Other miscellaneous causes of cerebral palsy include fetal anemia, twin-twin transfusion syndrome, intrauterine transfusions, and fetal alcohol syndrome (DeJong, 2012; Lindenburg, 2013; O’Leary, 2012; Rossi, 2011; Spruijt, 2012).
TABLE 33-2. Perinatal Risk Factors Reported to Be Increased in Children with Cerebral Palsy
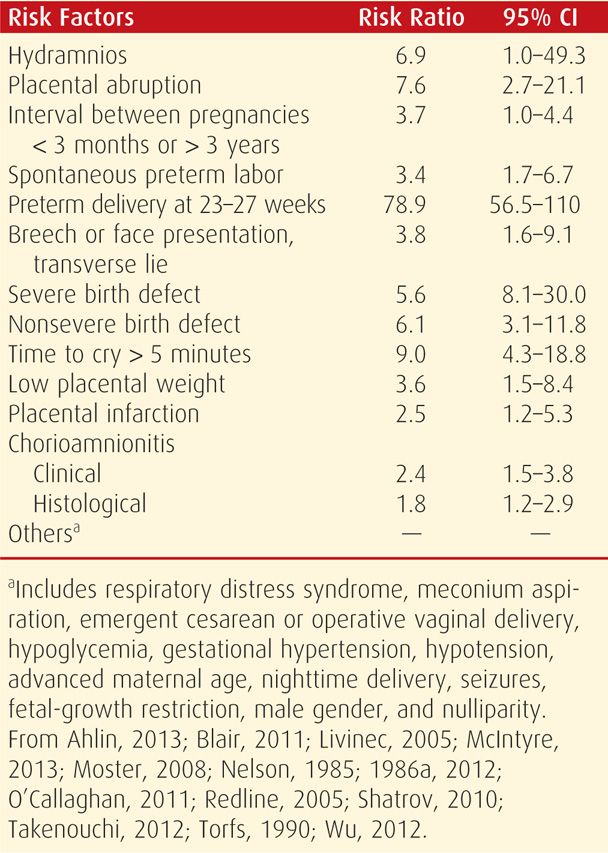
Intrapartum Events. The National Collaborative Perinatal Project found that intrapartum hypoxemia was linked to a minority of cerebral palsy cases. However, because the study was carried out in the 1960s, there were inconsistent criteria to accurately assign cause. The contribution of hypoxic-ischemic encephalopathy to subsequent neurological disorders is discussed in detail on page 639. Thus, the 2003 Task Force applied these criteria to more contemporaneous outcomes and determined that only 1.6 cases of cerebral palsy per 10,000 deliveries are attributable solely to intrapartum hypoxia. This finding is supported by a study from Western Australia that spanned from 1975 to 1980 (Stanley, 1991). These latter investigators concluded that intrapartum injury as the cause of cerebral palsy was unlikely in 92 percent of cases; in 3 percent, it was possible; and in only 5 percent was it likely. In another review of 209 infants with neurological impairment, 75 percent were classified as nonpreventable (Phelan, 1996). In yet another study of 213 such children, only 2 percent of cases could be attributed to intrapartum hypoxia (Strijbis, 2006).
Intrapartum Fetal Heart Rate Monitoring
Despite persistent attempts to validate continuous intrapartum electronic fetal monitoring as effective to prevent adverse perinatal outcomes, evidence does not support its ability to predict or reduce cerebral palsy risk (Clark, 2003; Devoe, 2011; MacDonald, 1985; Thacker, 1995). Importantly, there are no specific fetal heart rate patterns predictive of cerebral palsy, and no relationship has been found between the clinical response to abnormal patterns and neurological outcome (Melone, 1991; Nelson, 1996). Indeed, an abnormal heart rate pattern in fetuses that ultimately develop cerebral palsy may reflect a preexisting neurological abnormality (Phelan, 1994). Because of these studies, the American College of Obstetricians and Gynecologists (2013a) has concluded that electronic fetal monitoring does not reduce the incidence of long-term neurological impairment.
Apgar Scores
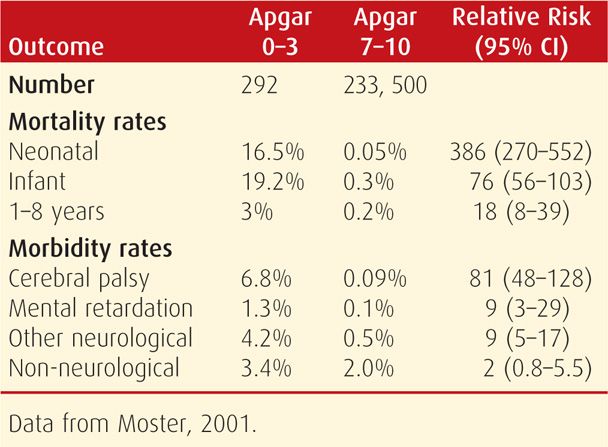
In general, 1- and 5-minute Apgar scores are poor predictors of long-term neurological impairment. When the 5-minute Apgar score is 3 or less, however, neonatal death or the risk of neurological sequelae is increased substantially (Dijxhoorn, 1986; Nelson, 1984). In a Swedish study, 5 percent of such children subsequently required special schooling (Stuart, 2011). In a Norwegian study, the incidence of these low Apgar scores was 0.1 percent in more than 235,000 newborns. Almost a fourth died, and 10 percent of survivors developed cerebral palsy (Moster, 2001).
Persistence past 5 minutes of these extremely low scores correlates strongly with an increased risk for neurological morbidity and death (Grünebaum, 2013). This of course is not absolute, and the 2003 Task Force cited a 10-percent risk for cerebral palsy for infants with 10-minute scores of 0 to 3. For 15-minute scores ≤ 2, there is a 53-percent mortality rate and a 36-percent cerebral palsy rate, and for 20-minute scores ≤ 2, there is a 60-percent mortality rate and a 57-percent rate of cerebral palsy. Some outcomes in the Norwegian Study of infants with these low 5-minute Apgar scores are shown in Table 33-3. Survivors with Apgar scores of 0 at 10 minutes have even worse outcomes. In a review of 94 such infants, 78 died, and all survivors assessed had long-term disabilities (Harrington, 2007).
TABLE 33-3. Comparison of Mortality and Morbidity in Norwegian Infants Weighing > 2500 g According to 5-Minute Apgar Scores
Umbilical Cord Blood Gas Studies
As outlined on page 639, objective evidence for metabolic acidosis—cord arterial blood pH < 7.0 and base deficit ≥ 12 mmol/L—is a risk factor for encephalopathy as well as for cerebral palsy. The risk increases as acidosis worsens. From their review of 51 studies, Malin and coworkers (2010) found that low cord arterial pH correlates with increased risk for neonatal encephalopathy and cerebral palsy. When used alone, however, these determinations are not accurate in predicting long-term neurological sequelae (Dijxhoorn, 1986; Yeh, 2012).
Data from several studies corroborate that a pH < 7.0 is the threshold for clinically significant acidemia (Gilstrap, 1989; Goldaber, 1991). The likelihood of neonatal death increases as the cord artery pH falls to 7.0 or less. Casey and colleagues (2001) reported that when the pH was 6.8 or lower, the neonatal mortality rate was increased 1400-fold. When the cord pH was ≤ 7.0 and the 5-minute Apgar score was 0 to 3, there was a 3200-fold increased risk of neonatal death.
In the study from Oxford, adverse neurological outcomes were 0.36 percent with pH < 7.1 and 3 percent with pH < 7.0 (Yeh, 2012). As mentioned, newborn complications increase coincident with increasing severity of acidemia at birth. According to the American College of Obstetricians and Gynecologists (2012d), encephalopathy develops in 10 percent of newborns whose umbilical arterial base deficit is 12 to 16 mmol/L, and in 40 percent of those whose deficit is > 16 mmol/L. In a Swedish study, researchers observed that cord blood lactate levels may prove to be superior to base deficit for prognostication of neurological disorders (Wiberg, 2010).
Nucleated Red Blood Cells and Lymphocytes
Both immature red cells and lymphocytes enter the circulation of term infants in response to hypoxia or hemorrhage. During the past two decades, quantification of these cells has been proposed as a measure of hypoxia, but most studies do not support this premise (Hankins, 2002; Silva, 2006; Walsh, 2011, 2013). The 2003 Task Force concluded that their use was investigational.
 Neuroimaging Studies in Encephalopathy and Cerebral Palsy
Neuroimaging Studies in Encephalopathy and Cerebral Palsy
Various neuroimaging techniques have provided important insight into the etiology and evolution of perinatal hypoxic-ischemic encephalopathy and later cerebral palsy (p. 639). Importantly, findings are highly dependent on fetal age. The preterm neonatal brain responds quite differently to an ischemic episode compared with that of a term infant. Other factors include insult severity and duration as well as restoration of cerebrovascular hypoperfusion. Thus, precise timing of an injury with neuroimaging studies is not a realistic goal. Reports are of two types—those in which neuroimaging was done during the neonatal period and those done when older children are diagnosed with cerebral palsy.
Neuroimaging in Neonatal Period
Regarding early use, the 2014 Task Force concluded that these imaging techniques provide the following information:
1. Sonographic studies are generally normal on the day of birth. Increasing echogenicity in the thalami and basal ganglia is seen beginning at approximately 24 hours. This progresses over 2 to 3 days and persists for 5 to 7 days.
2. Computed tomography scans are usually normal the first day in term infants. Decreased density in the thalami or basal ganglia is seen beginning at about 24 hours and persists for 5 to 7 days.
3. Magnetic resonance imaging will detect some abnormalities on the first day. Within 24 hours, MR imaging may show restricted water diffusion that peaks at about 5 days and disappears within 2 weeks. Acquisitions with T1- and T2-weighted images show variable abnormalities, which have an onset from less than 24 hours to several days. In a study of 175 term infants with acute encephalopathy, it was reported that MR imaging showing basal ganglia lesions accurately predicted motor impairment at 2 years of age (Martinez-Biarge, 2012).
The 2014 Task Force concluded that for term infants, imaging studies are helpful in timing an injury, but they provide only a window in time that is imprecise. Findings in preterm infants are considered in Chapter 34 (p. 656).
Neuroimaging in Older Children with Cerebral Palsy
Imaging studies performed in children diagnosed with cerebral palsy frequently show abnormal findings. Wu and associates (2006) used CT or MR imaging to study 273 children who were born after 36 weeks and who were diagnosed later in childhood with cerebral palsy. Although a third of these studies were normal, focal arterial infarction was seen in 22 percent; brain malformations in 14 percent; and periventricular white-matter injuries in 12 percent. In another study of 351 children with cerebral palsy—about half were born near term—MR imaging findings were abnormal in 88 percent (Bax, 2006). Similar findings were reported in an Australian study (Robinson, 2008).
CT and MR imaging techniques have also been used in older children in an attempt to define the timing of fetal or perinatal cerebral injury. Wiklund and coworkers (1991a,b) studied 83 children between ages 5 and 16 years who were born at term and who developed hemiplegic cerebral palsy. Nearly 75 percent had abnormal CT findings, and these investigators concluded that more than half had CT findings that suggested a prenatal injury. Approximately 20 percent was attributed to a perinatal injury. In a similar study, Robinson and colleagues (2008) used MR imaging. They reported pathological findings in 84 percent of children with spastic quadriplegia, which is the neurological lesion that the 2014 Task Force reported to correlate with neonatal encephalopathy.
 Intellectual Disability and Seizure Disorders
Intellectual Disability and Seizure Disorders
The term intellectual disability is the preferred substitute term for the older culturally insensitive term mental retardation (Centers for Disease Control and Prevention, 2012). It describes a spectrum of disabilities and seizure disorders that frequently accompany cerebral palsy. But when either of these manifests alone, they are seldom caused by perinatal hypoxia (Nelson, 1984, 1986a,b). Severe mental retardation has a prevalence of 3 per 1000 children, and its most frequent causes are chromosomal, gene mutations, and other congenital malformations. Finally, preterm birth is a common association for these (Moster, 2008).
The major predictors of seizure disorders are fetal malformations—cerebral and noncerebral; family history of seizures; and neonatal seizures (Nelson, 1986b). Neonatal encephalopathy causes a small proportion of seizure disorders. Reports from the Neonatal Research Network and other studies concluded that increasing severity of encephalopathy correlates best with seizures (Glass, 2011; Kwon, 2011).
Autism Spectrum Disorders
According to the Centers for Disease Control and Prevention (2012), the frequency of autism is almost 0.5 percent, and for attention deficit hyperactivity disorders, it is 6.7 percent. Although these may be associated with maternal metabolic conditions, none has been linked convincingly to peripartum events (Krakowiak, 2012).
HEMATOLOGICAL DISORDERS
There are a few neonatal disorders of erythrocytes, platelets, and coagulation with which the obstetrician should be familiar. As is the case for most other conditions manifest by the newborn shortly after birth, many of these hematological problems were acquired by the fetus and persist in the newborn.
 Anemia
Anemia
After 35 weeks’ gestation, the mean cord hemoglobin concentration is approximately 17 g/dL, and values below 14 g/dL are considered abnormal. A review of nearly 3000 deliveries found that late cord clamping was associated with a mean hemoglobin increase of 2.2 g/dL (McDonald, 2008). Unfortunately, this practice almost doubled the incidence of hyperbilirubinemia requiring phototherapy. The American College of Obstetricians and Gynecologists (2012b) has concluded that optimal timing of cord clamping for term infants is unknown. Conversely, cord blood hemoglobin concentration may be abnormally low, or it may fall after delivery. Fetal anemia results from many causes and is discussed in Chapter 15 (p. 306).
Acute anemia with hypovolemia is seen with deliveries in which the placenta is cut or torn, if a fetal vessel is perforated or lacerated, or if the infant is held well above the level of the placenta for some time before cord clamping. Intracranial or extracranial injury or trauma to fetal intraabdominal organs can also cause hemorrhage with acute anemia (Akin, 2011).
 Polycythemia and Hyperviscosity
Polycythemia and Hyperviscosity
Neonatal polycythemia with hyperviscosity can be associated with chronic hypoxia in utero, twin-twin transfusion syndrome, placental- and fetal-growth restriction, fetal macrosomia from maternal diabetes, and transfusion at delivery. Current recommendations by the International Liaison Committee on Resuscitation (ILCOR) are to delay cord clamping for at least 1 minute after delivery (Davis, 2012). When the hematocrit rises above 65, blood viscosity markedly increases and may cause neonatal plethora, cyanosis, or neurological aberrations. Because of the shorter lifespan of macrocytic fetal erythrocytes, hyperbilirubinemia commonly accompanies polycythemia as subsequently discussed. Other findings include thrombocytopenia, fragmented erythrocytes, and hypoglycemia. Partial exchange transfusion may be necessary in some neonates.
 Hyperbilirubinemia
Hyperbilirubinemia
Even in term fetuses, hepatic maturation is not complete, and thus some unconjugated bilirubin—either albumin bound or free—is cleared by placental transfer to be conjugated in the maternal liver (Chap. 7, p. 141). Fetal protection from unconjugated bilirubin is lost after delivery if it is not cleared rapidly. Because clearance is totally dependent on neonatal hepatic function, varying degrees of neonatal hyperbilirubinemia result. Even in the mature newborn, serum bilirubin usually increases for 3 to 4 days to reach levels up to 10 mg/dL. After this, concentrations usually fall rapidly. In one large study, 1 to 2 percent of neonates delivered at 35 weeks’ gestation or later had a maximum serum bilirubin level > 20 mg/dL (Eggert, 2006). In approximately 15 percent of term newborns, bilirubin levels cause clinically visible skin color changes termed physiological jaundice (Burke, 2009). As expected, in preterm infants, the bilirubin increase is greater and more prolonged.
Acute Bilirubin Encephalopathy and Kernicterus
Excessive serum bilirubin levels can be neurotoxic for newborns (Dijk, 2012; Watchako, 2013). The pathogenesis is complex, and toxicity has two forms. Acute bilirubin encephalopathy is encountered in the first days of life and is characterized by hypotonia, poor feeding, lethargy, and abnormal auditory-evoked responses (Kaplan, 2011). Immediate recognition and treatment will usually mitigate progressive neurotoxicity. The chronic form is termed kernicterus—from Greek jaundice of the nuclei. Neurotoxicity follows bilirubin deposition and staining of the basal ganglia and hippocampus and is further characterized by profound neuronal degeneration. Survivors have spasticity, muscular incoordination, and varying degrees of mental retardation. Although there is a positive correlation between kernicterus and unconjugated bilirubin levels above 18 to 20 mg/dL, it can develop at much lower concentrations, especially in very preterm neonates (Sgro, 2011). Continuing hemolysis is a risk factor for kernicterus, and experiences from the Collaborative Perinatal Project were that bilirubin levels ≥ 25 mg/dL correlated with kernicterus only with ongoing Coombs-positive hemolysis (Kuzniewicz, 2009). This was recently verified in a study by Vandborg and associates (2012).
Prevention and Treatment
Various forms of phototherapy are used to prevent as well as treat neonatal hyperbilirubinemia (Hansen, 2011). These “bili-lights” emit a spectrum of 460 to 490 nm, which increases bilirubin oxidation to enhance its renal clearance and lower serum levels. Light that penetrates the skin also increases peripheral blood flow, which further enhances photo-oxidation. It is problematic that available devices are not standardized (Bhutani, 2011). Another advantage is that exchange transfusions are seldom required with phototherapy. There are studies in both preterm and term newborns that attest to phototherapy efficacy (Watchko, 2013). A Neonatal Research Network study reported that aggressive phototherapy in low-birthweight neonates reduced rates of neurodevelopmental impairment (Newman, 2006).
For term infants, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012b) stress early detection and prompt phototherapy to prevent bilirubin encephalopathy. Despite these measures, bilirubin encephalopathy persists, and this is somewhat related to early hospital discharges (Gazzin, 2011; Kaplan, 2011; Sgro, 2011). According to Burke and coworkers (2009), hospitalizations for kernicterus in term infants were 5.1 per 100,000 in 1988. Since then, however, this rate has decreased to 0.4 to 2.7 cases per 100,000 births (Watchko, 2013).
 Hemorrhagic Disease of the Newborn
Hemorrhagic Disease of the Newborn
This disorder is characterized by spontaneous internal or external bleeding beginning any time after birth. Most hemorrhagic disease results from abnormally low levels of the vitamin K-dependent clotting factors—V, VII, IX, X, prothrombin, and proteins C and S (Zipursky, 1999). Infants whose mothers took anticonvulsant drugs are at higher risk because these suppress maternal hepatic synthesis of some of these factors (Chap. 60, p. 1190). Classic hemorrhagic disease is usually apparent 2 to 5 days after birth in infants not given vitamin K prophylaxis at delivery (Busfield, 2013). Delayed hemorrhage may occur at 2 to 12 weeks in exclusively breast-fed infants because breast milk contains little vitamin K. Other causes of neonatal hemorrhage include hemophilia, congenital syphilis, sepsis, thrombocytopenia purpura, erythroblastosis, and intracranial hemorrhage.
The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) recommends routine prophylaxis for hemorrhagic disease with a 0.5 to 1 mg dose of vitamin K1 (phytonadione) given intramuscularly. Oral administration is not effective, and maternal vitamin K administration results in very little transport to the fetus. For treatment of active bleeding, vitamin K is injected intravenously.
 Thrombocytopenia
Thrombocytopenia
Abnormally low platelet concentrations in term newborns may be due to various etiologies such as immune disorders, infections, drugs, or inherited platelet defects, or they may be part of a congenital syndrome. In many, thrombocytopenia is an extension of a fetal disorder such as infection with B19 parvovirus, cytomegalovirus, toxoplasmosis, and others discussed in Chapters 64 and 65. Term infants admitted to neonatal intensive care units, especially those with sepsis, have accelerated platelet consumption (Eissa, 2013). In these, transfusions are occasionally indicated (Sallmon, 2012).
Immune Thrombocytopenia
In women with an autoimmune disorder such as systemic lupus erythematosus or immunological thrombocytopenia, maternal antiplatelet IgG is transferred to the fetus and can cause accelerated platelet destruction. Most cases are mild, and platelet levels usually reach a nadir at 48 to 72 hours. Maternal corticosteroid therapy generally has no effect on fetal platelets. Fetal blood sampling for platelet determination is seldom necessary, and platelets are usually adequate to prevent fetal hemorrhage during delivery (Chap. 56, p. 1115).
Alloimmune Thrombocytopenia
Alloimmune thrombocytopenia (AIT) or neonatal alloimmune thrombocytopenia (NAIT) is caused by maternal-fetal platelet antigen disparity. If maternal alloimmunization is stimulated, then transplacental antiplatelet IgG antibodies cause severe fetal thrombocytopenia, which is considered in detail in Chapter 15 (p. 313).
Preeclampsia Syndrome
Maternal platelet function and destruction can be severely affected in women with severe preeclampsia. That said, fetal or neonatal thrombocytopenia is rarely caused by the preeclampsia syndrome even when the mother has severe thrombocytopenia. The large study of mother-infant pairs delivered at Parkland Hospital dispelled earlier reports of an association of neonatal thrombocytopenia with preeclampsia (Pritchard, 1987). Instead, neonatal thrombocytopenia was found to be associated with preterm delivery and its myriad complications (Chap. 34, p. 653).
INJURIES OF THE NEWBORN
Birth injuries can potentially complicate any delivery. Thus, although some are more likely associated with “traumatic” delivery by forceps or vacuum, others are seen with otherwise uncomplicated vaginal or cesarean delivery. In this section, some injuries are discussed in general, but specific injuries are described elsewhere in connection with their associated obstetrical complications, for example, breech delivery in Chapter 28 or multifetal gestation in Chapter 45.
 Incidence
Incidence
In three population studies that included more than 8 million term infants, the overall incidence of birth trauma was 20 to 26 per 1000 deliveries (Baskett, 2007; Linder, 2012; Moczygemba, 2010). Of these studies, data from Nova Scotia are shown in Table 33-4 with an overall trauma risk of 19.5 per 1000 deliveries. Only 1.6 per 1000 were due to major trauma, and these rates were highest with failed instrumented delivery and lowest with cesarean delivery without labor. Thus, most traumatic injuries were minor and had an incidence of 18 per 1000 deliveries.
TABLE 33-4. Incidence of Major and Minor Birth Trauma—Nova Scotia, 1988–2001
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


