25–34 yo); low socioeconomic status; urban location (JAMA 2001;285:1186).
Pathophysiology
• HIV RNA virus targets CD4 receptor on T-lymphocytes
• Destruction & impairment of CD4 cells → immunodeficiency → OIs thrive
• Monit dz progression & resp to rx w/ CD4 count & viral RNA-load
• Potentiation of transmission by other STIs. Infxn w/ STI ↑ HIV risk 2–5× due to ↑ viral shedding, genital mucosal disruption, & local recruitment of inflamm cells (Curr Opin HIV/AIDS 2010;5:305); includes HSV, BV, trichomonas, gonorrhea/chlamydia & HPV
Gynecologic Care (Obstet Gynecol 2010;116:1492)
• HIV screening recommended: IV drug use, HIV+ sex partner, STI dx, prostitution, multi sex partners, Preg
• 1st-step screening by ELISA → Western blot for band specific confirmation
• HIV+ → ↑ number & severity of vaginal infections → screen frequently for other STIs
• Clinical course differs w/ HIV coinfection. HSV → ↑ frequency, pain, duration; use HSV suppression ppx. Syphilis → ↑ neurosyphilis & rx failure; re-evaluate clinically & w/ serologic titers at 3, 6, 9, 12, & 24 mo after therapy (CDC MMWR 2010;59:No.RR-12)
• Latex condoms are the only contraceptive that reduces HIV transmission; spermicides do NOT reduce transmission.
• HAART recommended for all HIV-infected individuals
• ↓ OCP efficacy w/ PIs & NNRTIs. Long-acting reversible contraception (IUD, implant) safe & effective.
• HIV+  6× greater odds of ↓ bone mineral density & 4× ↑ odds of osteoporosis
6× greater odds of ↓ bone mineral density & 4× ↑ odds of osteoporosis
• ↑ incid of abn cervical cytology. 4–6× ↑ risk for CIN. HPV infxn = 65% in HIV+ women vs. 30% seronegative (JAMA 2000;283:1031; Glob Libr Women’s Med 2009;10:3843) w/ ↑ HPV persistence & progression. Incid of CIN correlates w/ ↓ CD4 count & ↑ HIV RNA levels. Routine colposcopy not recommended.
• Cervical cancer ↑ due to behavioral (less screening, IV drug use) & biologic factors (immunosuppression). More likely to present at advanced clinical stage.
• VIN, VAIN, & AIN also ↑ in HIV+ women (Obstet Gynecol 2006;107:1023)
• HAART a/w ↓ prevalence, ↓ incid & ↑ clearance of SIL (J Inf Dis 2010;201:681)
HIV in Pregnancy (http://aidsinfo.nih.gov/guidelines)
• Univ routine testing (opt-out) for all pregnant women at initial prenatal visit. Women who present in labor w/o prenatal care should get rapid HIV test; intrapartum AZT ↓ perinatal transmission. HIV a/w SGA, preterm deliv.
• Due to Preg plasma vol changes, CD4 count ↓ but no change on CD4 percentage. Dz progression unusual Preg (J infect Dis 1992;165:1116)
• HIV+ women should get pneumococcal, influenza, hepatitis A (if nonimmune), & hepatitis B vaccines + other std Preg vaccination. Screen for hepatitis C, given high rates of coinfection (MMWR Recomm Rep 2009;58:1).
• Transmission can occur transplacentally (related to mat viral load), during deliv, & w/ breastfeeding (N Engl J Med 1999;341:1698). HAART can ↓ perinatal transmission to <1% (untreated 15–25%) (N Engl J Med 1994:331:1173). Start HAART during Preg to suppress viral load, continue ppx at deliv, & provide neonat ppx to the infant.
• Transmission rates: HAART < dual therapy < AZT monotherapy << no therapy
Antepartum: All women should receive HAART during Preg – generally a combination from at least 2 classes of drugs. Recommended regimen is Zidovudine/Lamivudine/Ritonavir/Lopinavir. Efavirenz (NNRTI) category D: A/w increased neural tube defects. Some women may opt to start HAART after 1st trimester & organogenesis.
Intrapartum AZT mgmt: AZT at onset of labor 2 mg/kg loading dose followed by 1 mg/kg/h until deliv. Optional for women on HAART w/ HIV viral load <400 copies/mL. Continue oral HAART intrapartum. Avoid artificial rupture of membranes & instrumentation (scalp electrodes, operative deliv) if poss.
Postpartum: Infants should receive AZT for 6 w. Infants born to mothers not on HAART should receive 3 doses of nevirapine. Mat HAART continuation is essent given high rates of nonadherence & subseq mortality postpartum.
Mode of deliv: CD ↓ transmission rates in women NOT receiving HAART & zidovudine monotherapy (2–4×). No signif difference in transmission rates btw CD & VD in women on HAART. CD indicated if viral load >1000 copies/mL. 3 h of AZT should be administered prior to operation if poss. Duration of ROM a/w transmission in women w/ unsuppressed viral load → best to perform CCD prior to ROM or active labor.
Breastfeeding not recommended in developed countries even when mother on HAART, due to postnatal transmission risk (MMWR Morb Mortal Wkly Rep 1985;34:721). Rate of HIV transmission ∼10% from breastfeeding, but varies based on mat CD4 count, HIV viral load, & HAART use. In developing world, do recommend breastfeeding b/c infant mortality from HIV offset by increased diarrheal & PNA illness in formula-fed infants (JAMA 2006; 296:794).
TORCH INFECTIONS
• T-oxoplasmosis, O-ther (Syphilis, Varicella, Parvo), R-ubella, C-ytomegalovirus, H-erpes simplex virus
• Infections classically transmitted uteroplacetentally or during deliv
• General rule: ↑ gestational age @ time of infxn = ↑ transmission rate
• Rubella & syphilis routinely screened in Preg, others if indicated by Hx/risk factors
• Most carry risk of IUFD, prematurity, growth restriction in addition to congen defects
Toxoplasmosis (Clin Infect Dis 1994:18:853; Clin Infect Dis 2008;47:554)
• Epidemiology: ∼38% of women have immunity. Incident infxn during Preg is 0.2–1%. Congen infxn due to re-infection rare. Congen toxoplasmosis incid 1–2 cases out of 100000.
• Microbiology: Toxoplasmosis gondii: A ubiquitous protozoan parasite. Life cycle: Cat (definitive host of parasite) intestines produce oocysts which produce sporozoites → passed in feces → animals eat sporozoites → cysts form in bone & muscle → humans eat raw, undercooked meat, consume the tissue → infxn. OR, humans ingest oocysts while handling cat litter or soil.
• Maternal–fetal transmission occurs during active phase of new infxn. Transmission rate btw around 30%. Likelihood of transmission ↑ w/ gestational age – 15% at 13 w, 44% at 26 w, 71% at 36 w. Severity of congen infections peaks during transmission around 24–30 w.
• Clinical manifestations: Mat usually subclinical or nonspecific (fever, malaise, LAD, myalgia). Fetal classic triad of chorioretinitis, hydrocephalus, intracranial calcifications. Also seizures, jaundice, HSM, anemia. Late manifestations, ocular, & neurologic (developmental delay). Subclinical dz more common – only 10% show signs of congen infxn.
• Dx/screening: No univ screening. Dx by mat serology. A single bld test does not distinguish btw acute & chronic infxn. Nor does IgM vs. IgG distinguish, as both persist in chronic infxn. Rising titers demonstrate new infxn → 4× ↑ or greater done at least 2 w apart (stable titers = chronic infxn which poses no risk to fetus). Once new infxn documented: PCR of amniotic fluid. US surveillance of fetal dev & manifestations of infxn.
• Rx (Lancet 2004;363:1965): Spiramycin (1 g TID) or pyrimethamine & sulfadiazine w/ leucovorin (teratogenic risk in 1st trimester w/ latter combo). Rx reduces serious neurologic sequelae. Unclear if rx prevents transmission & ocular sequelae.
• Prevention: Hand hygiene, avoiding uncooked meats, cats, unfiltered water, & travel to less developed countries
Syphilis (see below)
Varicella virus (VZV) (BJOG 2011;118:1155)
• Epidemiology: 90% of women are infected (chickenpox) before adulthood. VZV incid in Preg ∼5/10000.
• Microbiology: Herpes virus responsible for 1° infxn known as VZV (chickenpox) w/ subseq reactivations known as zoster. Mat zoster (shingles) rarely a/w congen VZV syn.
• Clinical manifestations (Obstet Gynecol 1987;69:214): Mat VZV infxn can be sev. VZV PNA = common complication (10–20%) w/ 20–40% mortality w/o antiviral therapy. Congen syn rare <2%. Transplacental transmission <20 w gestation characterized by limb hypoplasia, cutaneous scars, neurologic abnormalities, ocular abnormalities, high mortality. After 20 w transmission, neonat dz 1° infxn near term (w/i 5 d of deliv) neonat mortality as high as 30%. Infxn >5 d from deliv, mat Ab xfer → more benign neonat infxn.
• Dx/screening: Mother: Characteristic vesicular papules in different stages of progression. Culture or immunofluorescence studies. Serology early in infxn can confirm mat nonimmunity. PCR testing of amniotic fluid + US to detect fetal infxn.
• Rx: 1° chickenpox or exposed & VZV IgG negative: Antiviral therapy w/i 24 h of rash appearance. Acyclovir 800 mg 5× daily or Valacyclovir 1 g TID for 7 d. VZV zoster secondary infxn: Ig w/i 72 h of exposure. May be effective up to 10 d after exposure. Pregnant women w/ varicella PNA should be admitted & treated w/ IV acyclovir.
Parvovirus (N Engl J Med 1987;316:183; Prenat Diagn 2011;31:419)
• Epidemiology: Incid of acute parvovirus in Preg = 3%. By adulthood 30–60% of women have had infxn.
• Microbiology (Rev Med Virol 2003;13:347): Risk of vertical transmission to fetus ∼33%. Virus affects fetal erythroid progenitor cells.
• Clinical manifestations: Children & adults → erythema infectiosum: Lace-like rash often on face “slapped check,” arthropy, aplastic anemia. Fetal infxn: Hydrops & stillbirth <24 w; >24 w, persistent risk of hydrops, but ↓ likelihood of sev infxn & death. Hydrops from anemia → reduced survival of fetal red cells → high-output CHF.
• Dx/screening: Exposed women should be tested w/ serology: + IgM w/o IgG = acute infxn. PCR amniotic fluid + US to confirm dx
• Rx: W/ confirmed infxn → surveillance for up to 12 w. Weekly US & MCA dopplers to look for fetal anemia. Intrauterine xfusion can be done to correct fetal anemia & ↓ fetal mortality.
Rubella (Lancet 1982;2:781; Glob Libr Women’s Med 2012)
• Epidemiology: Rare in the US given immunization programs. ∼90% of pop immune
• Congen rubella extremely rare – <1 case/y in US recently
• Microbiology: Self-limited viral infxn transmitted in droplets or nasopharyngeal secretions from infected persons, commonly from contact w/ infected child. Congen infxn occurs via hematogenous spread across placenta. Earlier transmission = higher likelihood of sev defects.
• Clinical manifestations: Mat often subclinical: Fever, desc maculopapular rash, LAD (post auricular), URI-like, nonspecific sx. Infxn in 1st trimester usually results in miscarriage. Infxn after 20 w unlikely to result in neonat manifestations. Classic fetal syn: Growth restriction, cataracts, cardiac defects, hearing defects, hepatosplenomegaly. Late manifestations: DM, thyroid disorders, panencephalitis.
• Dx/screening: Univ screening at initial prenatal visit → nonimmune pts vaccinated postpartum. Dx by serology titer immediately following exposure. If Ab + → woman likely immune, no risk to fetus. Conversion of (–)Ab or 4× ↑ titer indicates acute infxn (rpt titers 2–4 w apart). Confirm by IgM or direct PCR of fetal bld.
• Rx/prevention: Mat supportive measures. No rx exists for preventing transmission or for fetal infxn. Nonimmune mothers should be vaccinated postpartum. MMR vaccine should not be administered to pregnant women b/c of theoretical risk of transmission from live virus. Advised to avoid conception for 1 mo following vaccine
Cytomegalovirus (CMV) (Infect Dis Obstet Gynecol 2011;2011:1)
• Epidemiology: Most common congen infxn. Birth prevalence ∼0.5%. Seropositivity in childbearing women ∼58% in US. Risk factors: Low socioeconomic status (near 100%), non-White, multiparous. Most common infectious cause of sensorineural hearing loss.
• Microbiology: Herpes virus family, latent in numerous organs following infxn. Transmitted by close interpersonal contact including sexual contact & breastfeeding. Congen CMV: Transplacental transmission. Peripartum transmission does not harm dev of neonate.
• Transmission (Obstet Gynecol Surv 2010;65:736): 1° mat infxn, ∼35% transmission. More likely to cause fetal infxn & sequelae. Reactivation of latent virus: 1–2% transmission rate. Reinfection w/ different strain poss.
• Clinical manifestations: 1° CMV – asx or a/w a mononucleosis-like syn. Fetal infxn & sequelae more common at <20 w gestation. 90% are asx; 5–10% overtly symptomatic w/ 5% mortality; 50–60% w/ sev neurologic morbidity: Microcephaly, ventriculomegaly, chorioretinitis, HSM, sensorineural hearing loss. Late infxns a/w hepatitis, PNA, purpura, & thrombocytopenia.
• Dx/screening: Routine screening not currently recommended in US. Dx by seroconversion during Preg. IgM helpful for reactivated infxn. Low IgG avidity indicative of primary infxn (can perform avidity testing). Viral culture can be performed, but does not distinguish btw new & recurrent. US screening for anomalies should be performed in suspected case. If CMV infxn present → amniocentesis to detect fetal infxn.
• Prevention: Hygienic precautions: Washing hands, avoidance of close contact
• Rx: High-titer CMV Ig may ↓ transmission & fetal/neonat morbidity
Herpes Simplex Virus (HSV) (N Engl J Med 1997;337:509)
• Epidemiology: HSV-1 or HSV-2 seroprevalence in pregnant females up to 72%. Congen HSV very rare, 1 in 5000–20000. Seroconversion during Preg = 3.7% in women seronegative to both types.
• Microbiology: 50–70% of genital HSV caused by HSV-2. Genital HSV-1 ↑ due to oral–genital practices. Transmission can occur transplacentally (rare) or through contact w/ mother’s genital tract during labor/deliv. Mat 1∞ infxn (0.1% incid) a/w higher transmission rates at deliv than recurrent infxn (mat antibodies are protective).
• Clinical manifestations: 1° infxn: Fever, malaise, dysuria, tender inguinal, LAD, painful genital ulcers. Many pts have mild or subclinical presentation. Vesicles → crusting ulcers. Recurrent episodes vary in frequency, usually milder & shorter than 1° episode. Latency: Dorsal nerve roots btw episodes. Neonat HSV: Mucocutaneous involvement (∼45%), CNS dz (∼33%), dissem dz w/ multiorgan involvement (∼25%). Congen infxn extremely rare, can cause systemic dz w/ mortality >50%. Late trimester mat infections correlated w/ increased rates of preterm labor, preterm birth, & IUGR.
• Dx/screening: Univ screening not recommended. Dx by culture or PCR if lesion present. Serology can distinguish HSV type; IgM indicative of acute infxn.
• Rx (MMWR Recomm Rep 2010;59:1): 1° infxn: Acyclovir 400 mg TID × 7–10 d or Valacyclovir 1 g BID × 7–10 d. Recurrent infxn: Acyclovir 400 mg TID × 5 d or Valacyclovir 500 mg BID × 3 d. Suppressive therapy recommended for women w/ recurrent genital HSV from 36 w until deliv: Acyclovir 400 mg TID or Valacyclovir 500 mg BID. At time of deliv, careful exam of woman’s genital tract should be performed. Women w/ active lesions of vulva, vagina, or cervix should be offered CD. Lesions on buttocks, mons, thighs, or anus can be covered during deliv.
OTHER INFECTIONS IN PREGNANCY
Influenza (Obstet Gynecol 2010;115:717) See also Chap 13.
• Epidemiology: During flu pandemics (including 2009 H1N1), pregnant women ↑ mortality rate, ↑ hospitalization, ↑ ICU admission, & ↑ deaths
• Clinical manifestations: Influenza → critical illness in Preg & carries much higher mortality rate. Physiologic changes of Preg → less cardiopulmonary reserve & altered immune system. Transplacental transmission rare & insig. Mat illness may lead to premature deliv.
• Dx/screening: Rapid flu testing available in 15 min or less, but sens is fairly poor ∼63%. In pregnant & recently (<2 w) postpartum women, rx should be administered clinically. Do not await diagnostic results.
• Rx: Neuraminidase inhibitors. Ppx for exposed: Oseltamivir 75 mg daily × 10 d or Zanamivir 10 mg daily × 10 d. Rx: Oseltamivir 75 mg BID × 5 d or Zanamivir 10 mg BID × 5 d.
• Prevention: All pregnant women should receive inactivated influenza vaccination regardless of gestational age, & preferably by the beginning of flu season.
Hepatitis B Virus (HBV) And see Chap 15.
• Epidemiology: Prevalence ∼1% US & 15–20% endemic areas (SE Asia, China, sub-Saharan Africa). Major source of morbidity from hepatitis, cirrhosis, & HCC. 5–10% of acutely infected will become chronic carriers. In endemic areas, perinatal is primary form of transmission.
• Microbiology (JAMA 1985;253:1740): Maternal–fetal transmission primarily during deliv. Transmission 40–90% w/o ppx, much higher if HBeAg +. CD does not prevent transmission. Breastfeeding does NOT ↑ rate of transmission (Obstet Gynecol 2002;99:1049).
• Clinical manifestations: Mat 1° infxn: Abdominal pain, fever, N/V, jaundice. Almost all infected infants become chronic carriers, although infxn generally asx. Infected newborns have risk for liver dz later in life.
• Dx/screening: Dx by + surface Ag (HBsAg). Immunity is indicative of loss of HBsAg & appearance of HBsAb (Ab). IgM anti-HBc is indicative of primary infxn; sometimes only sign of infxn btw loss of HbsAg & rise of HBsAb. HBeAg a marker of high infectivity, carries high vertical transmission rates. Chronic infxn is determined by persistence of HBsAg >6 mo. ACOG recommends univ screening by checking HBsAg in prenatal panel.
• Rx: Vaccination universally recommended if serologically negative. Lamivudine during 3rd trimester may ↓ rate of transmission (Obstet Gynecol 2010;116:147). HepBIg & HBV vaccine recommended as ppx for neonates of HbsAg+ women. ↓ rate of transmission by almost 90% (JAMA 1985;253:1740).
Hepatitis C Virus (HCV) (Hepatology 2001;34:223; Am Fam Physician 2010;82:1225) See Chap 15.
• Epidemiology: 1.8% of noninstitutionalized persons carry HCV antibodies
• Microbiology: Vertical transmission ∼2% – primarily during deliv. Risk factors for increased transmission include increased viral load, HIV coinfection, mat drug use, prolonged ROM, procedures during labor (fetal scalp electrode, operative vaginal deliv). CD does not appear to lower rates (Arch Gynecol Obstet 2011;283:255). Breastfeeding does not to appear to ↑ transmission. Infected infants are generally asx, sometimes w/ temporary transaminitis.
• Dx/screening: Hepatitis C screening is not univ; based on risk factors. HCV Ab + → obtain viral load, genotype. HCV by RIBA if concern for false-positive.
• Rx: Std – combined pegylated interferon alfa-2a & ribavirin. Not safe rx during Preg, ribavirin = teratogenic. Vaccinate for HBV if not infected or immune.
Tuberculosis (TB) (Chest 1992;101:1114)
• Epidemiology: Same in Preg as general pop. Btw 5–10% of reproductive women have reactive tuberculin skin test. Worldwide TB = leading infectious dz cause of mat mortality. Risk factors in US: Low socioeconomic status, urban area, IV drug use, homelessness, immigrant from underdeveloped country, & incarceration.
• Clinical manifestations: 3–4% develop active TB during 1st year. 5–15% will later develop an active infxn. Active TB: Cough, fever, hemoptysis, weight loss, fatigue, night sweats. Untreated active infxn has a 50% mortality rate at 5 y. Active TB → congen infxn through transplacental transmission. Extremely rare & a/w miliary TB.
• Dx/screening: Screening should occur in women w/ risk of progression from latent to active. TST or interferon gamma release assay. Those w/ positive testing should undergo CXR.
• Rx (MMWR 2000;49:1):
Latent TB: 9 mo INH 5 mg/kg/d. Can delay rx of latent TB until 2–3 mo PP unless high risk of progression to active dz (HIV+, recent contacts)
Active TB: INH, rifampin, ethambutol ± pyrazinamide × 9 mo minimum. Streptomycin should be avoided in Preg (congen deafness). Breastfeeding not contraindicated during rx. Infant should be given pyridoxine (B6) if mother is on INH.
HUMAN PAPILLOMA VIRUS (HPV)
Epidemiology
• Most common sexually transmitted virus worldwide
• Most common viral cause of cancer worldwide (5% of all cancers)
• Worldwide prevalence around 10% although 80% of sexually active adults will acquire an HPV infxn in their lifetime (Am J Epidemiology 2000;151:1158)
• Prevalence highest in teenagers & young women shortly after sexual debut (JAMA 2007;297:813)
• Risk factors: Young age, early age at 1st intercourse, number of sexual partners, other STIs (HIV, HSV, chlamydia), smoking, low education, minority race
Microbiology
• DsDNA virus. ∼40 strains of HPV infect the anogenital tract & can be a/w anogenital warts & cancer including cervical, vaginal, vulvar, oropharyngeal, anorectal, & penile. High-risk HPV types cause cancer: HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68. Types 16 & 18 account for 70% of cancer cases (N Engl J Med 2003;348:518). Low-risk HPV types cause warts: HPV6, 11, 42, 43, 44.
• Transmission usually through intercourse, but can occur through close personal contact.
• Warts esp contagious → infectivity up to 60%. Vertical transmission may be as high as 55% during vaginal deliv. Infxn generally transient → 90% cleared by 24 mo (Vaccine 2006;24:S42).
• Risk factors for persistence/progression of HPV to precancerous lesions: Older age, immunosuppression, cigarette smoking, high-risk genotypes (Vaccine 2006;24:42).
• HPV’s carcinogenic potential related to (J Virol 1989;63:4417)
E6 gene a/w inactivation of p53 (tumor suppressor prot)
E7 gene a/w inactivation of the Rb apoptotic pathway
Clinical Manifestations
• Based on strain & site of infxn. Include genital & nongenital warts (condyloma acuminatum), Bowen’s dz (squamous carcinoma in situ), giant condyloma, & intraepithelial neoplasia.
• Cervical dysplasia: HPV infxn leading to cellular atypia & progression from low-grade to high-grade histology is the basis for cervical cancer (J Pathol 1999;189:12) See also Chaps 1 (screening) and 21 (cancer).
• Genital warts (condylomata acuminata): Caused by low-risk HPV 6 & 11 (90%) (MMWR Recomm Rep 2010;59:1). Usually asx papillomatous growths, commonly appear around introitus. Vary in appearance: Hyperpigmented, papilliform, flat, papular, pedunculated (in contrast to condylomata lata of syphilis which is flat & velvety). Regression occurs ∼20–50% of cases. Persistence a/w immunocomp status & dev of squamous cell carcinoma. Lesions a/w HPV 6 & 11 are almost 100% benign. 30% of flat condylomas a/w high-risk types & have oncogenic potential.
Diagnostic Studies
• HPV testing: Current testing exists in either a binary (± high-risk HPV) form or specific genotyping that can detect presence of specific strains (HPV16, HPV18). See Ch. 1 and 21.
• Genital warts: Dx of condyloma made by inspection. 5% acetic acid solution → causes acetowhite change for easier identification. Bx considered if dx uncertain, lesion does not respond to rx or worsens w/ therapy, pt immunocomp, warts are pigmented, indurated, fixed, bleeding, or ulcerated.
Treatment (MMWR Recomm Rep 2010;59:1)
• Rx goal for genital warts is amelioration of sx & cosmetic improv
• CDC recommended regimens:
Patient-applied:
Podofilox 0.5% gel applied BID × 3 d followed by 4 d off, up to 4 cycles
Imiquimod 5% cream applied qhs 3 × a week for up to 16 w
Sinecatechins 15% ointment TID for up to 16 w
5-fluorouracil 5% cream applied BID × 5 d followed by 9 d off, up to 4 cycles
(Safety of all of these therapies in Preg is unk & should not be used)
Provider-administered: Cryotherapy, trichloroacetic acid 85%, surgical removal (excision, laser, electrosurgery, infrared coagulation)
• Ppx (CDC-ACIP 2011)
Bivalent (Cervarix) & quadrivalent (Gardasil) vaccine. Quadrivalent includes HPV 16, 18 as well “low-risk” strains HPV 6, 11. Vaccination recommended in females & males 11–26 yo. Bivalent effective against HPV 16, 18.
SYPHILIS
Epidemiology (MMWR 2003;52:1117)
• Once highly prevalent dz now uncommon in US & developed countries.
• Increasingly common in MSM populations. Up to 25% coinfection rate w/ HIV – most prevalent in Africa, India, SE Asia, & the Caribbean.
Microbiology
• Caused by the spirochete: Treponema pallidum. Sexually transmitted through microabrasions of intercourse or vertical transmission. Following infxn, the organisms invade LN to disseminate to other organs.
• Sexual transmission occurs during both primary & secondary infxn. Rate ∼30%.
Requires open lesions w/ organisms present
Clinical Manifestations
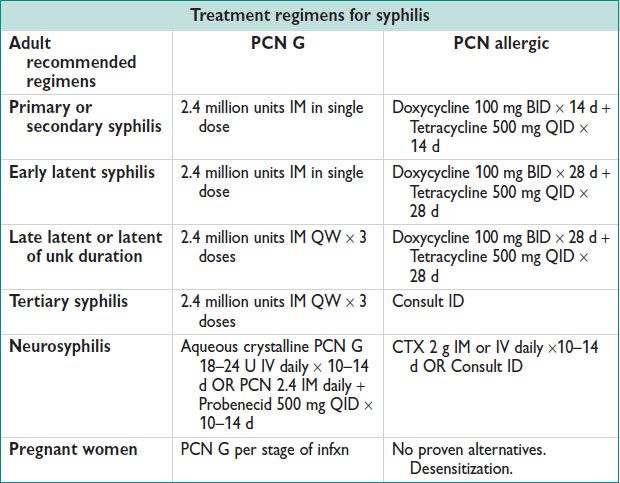
• Primary syphilis
Chancre at site of inoculation. Ulcer is usually single, painless, indurated, a/w LAD. Most common location in women: Labia majora + minora, fourchette, cervix, perineum. Generally heal w/o rx secondary to natural immune resp.
• Secondary syphilis
Weeks to months after primary infxn. ∼25% develop systemic illness.
Rash (typically palms & soles), up to 90% of pts. 0.5–2 cm in diameter often referred to as copper pennies.
Condyloma lata: 10–15% pts. Large raised white lesions usually near chancre
Highly infectious – not to be confused w/ condyloma acuminatum (HPV/warts)!
Systemic sx: Fever, HA, malaise, LAD.
Immunocomp can develop ocular dz, ulcerative lesions
• Latent syphilis
Asx w/ + serologic testing. If pt never had sx = latent of unk duration. Early/late distinction is <1 y (early latent) vs. >1 y (late latent) from sx. Pts >1 y from sx are relatively noninfectious.
• Tertiary syphilis (late)
Develops after latent period of 1–30 y after primary infxn. Most common manifestations: CNS (neurosyphilis), CV (aortitis), Gummatous (on skin & bones)
Neurosyphilis: Syphilitic meningitis (syphilis in CSF), meningovascular syphilis (ischemia/infarction of CNS), parenchymal syphilis (tabes dorsalis, general paresis)
• Congen syphilis
Preg does not change course of mat dz. Fetal manifestations depend on time of transmission. Early infxn → high rates of SAB. Late infxn → placental involvement, hydrops, IUGR, stillbirth. Transmission ↑ w/ GA, but severity ↓.
Vertical transmission highest w/ 1° or 2° syphilis (50%). Rx lowers risk of transmission to 1–2%. Congen syphilis is classified as early or late. Early: Sx prior to 2 yo: Rhinitis (snuffles), rash, PNA, HSM, osteochondritis (similar to adults). Late: Sx after 2 yo: Saddle nose, Hutchinson’s teeth (peg-shaped incisors), keratitis, deafness, gumma, skeletal & CNS malformations.
Diagnostic Studies
• Organism cannot be cultured. Definitive dx made by direct visualization of organisms w/ either dark field microscopy (gold std), direct fluorescent Ab, or PCR.
• Serologic testing: Nontreponemal (VDRL, RPR) vs. treponemal (FTA-ABS, TPA)
Nontreponemal tests – used for pop screening (Preg, MSM). Low cost, widely available. Use titers to monit resp to rx. 4-fold change in titer necessary to demonstrate resp to rx. False positives a/w Preg, autoimmune dz, IV drug use, TB, rickettsial infxn, hepatitis, malig. Sensitivities can be poor esp during primary & late stages (70–80%).
Treponemal tests – more specific, generally used for confirmatory testing. Cannot be used alone to diagnose rpt infxn as antibodies may stay positive following successful rx. False positives a/w lupus, Lyme’s, leptospirosis.
• Preg: Screening at the 1st prenatal visit w/ nontreponemal test followed by rpt testing in 3rd trimester & at deliv if pt is high risk.
• Lumbar puncture w/ signs of tertiary/neurosyphilis, or HIV+ & latent syphilis
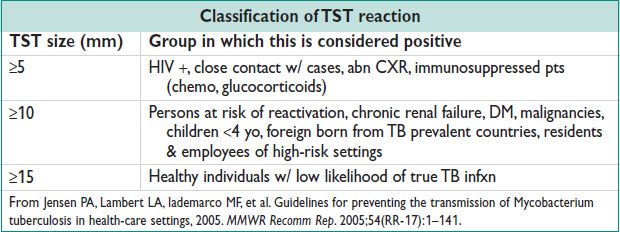
Treatment
• Jarisch–Herxheimer rxn: Febrile rxn w/i 24 h of rx → release of inflamm proteins from dead or dying organisms. Can induce preterm labor or cause fetal distress in pregnant women.
• Eval should be made at 6 & 12 mo after rx (24 if latent dz or worse). Serologic testing should show decline by 4 fold. Failure of decline = re-evaluation for HIV, CSF exam should be considered. Retreatment involves 2.4 million units IM for 3 w.
• Partners exposed w/i 90 d of dx & partners of pts w/ syphilis of unk duration & high nontreponemal titers (>1:32) should be treated presumptively (MMWR Recomm Rep 2010;59:1).
MOLLUSCUM CONTAGIOSUM
Epidemiology (J Am Acad Dermatol 2006; 54:47)
• Common worldwide. A/w childhood, immunodeficiency (including HIV) & atopic dermatitis. Seropositivity up to 25% in general pop.
Microbiology
• Pox virus spread through direct skin-to-skin contact or through fomites
• Considered a sexually transmitted infxn when found in genital region
Clinical Manifestations
• Firm dome-shaped papules on skin w/ shiny surface & central umbilication
Appear anywhere except palms & soles, but generally localized. Commonly in skin folds – axilla, popliteal folds. Sexually transmitted areas include groin, genitals, thighs, & lower abd. Dermatitis can occur around the lesion – erythema & pruritus.
• Widespread, large >15 mm lesions should raise suspicion for HIV+.
• Natural Hx in immunocompetent person: Spont resolution in months
Lesions can last years (Int J Dermatol 2006;45:93)
Diagnosis
• Clinical – based on appearance of lesion. Histology: H&E reveals Henderson-Patterson Bodies: keratinocytes w/ cytoplasmic inclusion bodies
Treatment
• No clear evid for rx given lesions are self-limiting. Rx of sexually transmitted lesions indicated to avoid transmission of dz. Perform comprehensive body exam to locate all lesions for rx.
• Cyrotherapy: Liquid nitrogen applied 6–10 s (can cause hypopigmentation)
• Other 1st-line options: Curettage, cantharidin, podophyllotoxin (Cochrane Database Syst Rev 2009)
CHANCROID
Epidemiology
• Uncommon in US & developed countries. Major cause of genital ulcers in developing countries. In US: Minority pop, female prostitutes, drug users. Up to 10% have concurrent syphilis infxn.
Microbiology
• Haemophilus ducreyi – gram negative rod (school of fish appearance). Extremely infectious. Incubation 3–10 d, reliant on break in skin. Cytotoxin secreted causes cellular damage & ulcer dev.
Clinical Manifestations
• Erythematous papule, 1–2 cm diameter → pustular & ulcerates. Distinguished from syphilis as it is painful, sometimes purulent & base is red & granular. Typically found on fourchette, vestibule, clitoris & labia (Clin Infect Dis 1997;25(2):292). Often single lesion but can be multi & bleeds.
• LAD present in ½ cases & can become fluctuant & painful.
Diagnosis (MMWR Recomm Rep 1990;39:1)
• Difficult lab dx due to need for culture on special media (sens <20%)
Special PCR test exists by private clinical labs. “Probable” dx based on clinical sx & negative testing for syphilis & HSV.
Treatment (MMWR Recomm Rep 2010;59:1)
• CDC recommendation: Azithromycin 1 g PO or CTX 250 mg IM or Ciprofloxacin 500 mg PO BID × 3 d. Ciprofloxacin contraindicated in pregnant & lactating women.
• Pt should be re-examined at 3–7 d after initiation of therapy. Lack of clinical improv w/i 7 d, consider incorrect dx, coinfection, HIV+, nonadherence, drugs resistance. Healing is slower for immunocompromised (HIV), uncirc men. LAD might require needle aspiration or drainage
PUBIC LICE
Epidemiology
• Generally transmitted sexually. Less commonly transmitted by fomites on clothing & bedding. Most commonly affected are teens, young adults.
Etiology
• Phthirus pubis or “crab louse” is primary organism. Crab-like claws attach to human hair, feeding on human bld, laying eggs. Eggs incubate for 6–8 d before hatching.
Clinical Manifestations
• Pruritus from attachment & biting
• Maculopapular lesion may develop (lower abd, prox thighs, buttocks)
• Manifestations can occur in any hairy area, but pubic area is often involved
Diagnostic Studies
• Demonstration of louse or nits (eggs) under microscopic exam
• Dx should trigger eval of family members, sexual contacts, & for other STIs.
Treatment (MMWR Recomm Rep 2010;59(RR-12):1)
• Pediculicides kill both lice & eggs
• CDC rec: Permethrin 1% cream or Pyrethrins w/ piperonyl butoxide (washed off after 10 min). Alternative: Malathion 0.5% (8–12 h) or Ivermectin (250 ug/kg) (for rx failure). Permethrin, Pyrethrin safe in pregnant & lactating women.
• Re-evaluate after 7–10 d of rx. Bedding or clothing should be bagged or washed.
Lice will die 48 h after removal from host or temperature >125°C.
GENITAL ULCERS
< div class='tao-gold-member'>



