Differential Cytology
Visual Analysis of the Cell Image
Benign Changes and How to Distinguish them from Malignancies
| 5 | Differential Cytology |
Visual Analysis of the Cell Image
The previous chapters have provided a systematic classification of cellular changes into normal, benign, and malignant categories. In day-to-day practice, however, it is often difficult to verify this classification in the gynecological smear, either because the morphological images are not typical enough, or degenerative, reactive, or proliferative changes exist side by side in the same smear. This creates problems with the identification and interpretation of atypical cells.
Whereas systematic cytology is concerned with the differences between normal, benign, and malignant morphologies, differential cytology focuses on their common features. Because of external and internal influences, benign and malignant lesions may yield cell images that resemble each other, thus leading to misinterpretation of such look alikes.
Changes responsible for problems of classification are subdivided into squamous epithelial, glandular epithelial, and small-cell lesions, although the transitions between them are fluid. Signs of dedifferentiation within the squamous epithelium include, for example, the presence of vacuoles and a loose, foamy cytoplasm with indistinct cell borders, whereas a loss of these features is observed with dedifferentiation in the columnar epithelium. It is not possible to specify a particular cell size that would indicate where large-cell differentiation ends and small-cell differentiation begins. Both these growth patterns often exist side by side. The diagnostic problem with small-cell lesions begins with a cell diameter of less than 14 μm.
Degenerative and reactive processes affect almost all cells of the smear, thus causing diffuse changes in the overall image of cells. However, neoplastic lesions preferentially manifest themselves in changes of single cells (or groups of cells) observed in the single cell image. The factors influencing the overall image and the specific morphology of single cell images may be so serious that benign cells appear abnormal whereas malignant cells do not raise any suspicion. Hence, the correct classification of such cells depends on the cytologist’s visual acuity, subjective evaluation, and morphological experience.
The interpretation of cellular changes is based on the detection of cytoplasmic and nuclear structures in the smear. Cytoplasmic features provide clues to the origin of a cell, and nuclear analysis helps with the assessment of its integrity. In order to classify the size and structure of problematic nuclei correctly, it is helpful to use clearly identifiable reference nuclei for orientation. These are nuclei of cells regularly present in a smear, such as intermediate cells or granulocytes. Technical, physiological, or inflammatory factors may alter both nuclei and cytoplasm to such an extent that only a few morphological criteria remain that make it possible to reach any conclusion about the origin of the cells and their degree of malignancy. The changes may be so extensive that an assignment is no longer possible.
The visual analysis of photographic images can be extremely difficult even for an experienced morphologist. Cell images that are clearly recognized in the cytological preparation are often not reproduced in enlarged sections of a microphotograph; first, because any reference to the overall image of cells is missing and, second, because it is not possible to vary the optical magnification and the plane of view. For this reason, the use of photographs, slides, or digitized images is restricted to the purposes of continuing education in cytology. Hence, participation in cytological workshops, where a rich variation of certain topics is presented directly at the microscope and in a practice-oriented manner, cannot be replaced by written teaching material. On the other hand, a textbook, an atlas, or a slide quiz does have the advantage of presenting specific problems in a clear arrangement of text and illustrations, thus providing food for thought with respect to differential diagnosis.
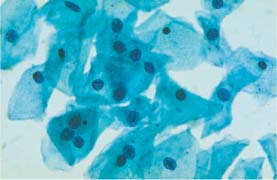
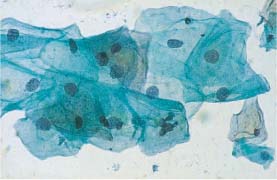
Fig. 5.1b Mild dysplasia. Irregular chromatin structure and irregular nuclear envelopes (DD: normal intermediate cells.) ×400.
Benign Changes and How to Distinguish them from Malignancies
Squamous Epithelial Lesions
The most common problems in differential cytology concern the squamous epithelium. The development of cervical carcinoma is correlated with high endemic HPV infection, which affects a large section of the population, mainly during sexual maturity. Furthermore, inflammatory genital lesions predominate during this period of life and may yield similar cell images.
Portio and Vagina
Physiological Factors
Cytolysis caused by lactobacilli not only lyses the cytoplasm but often results in the edematous swelling of the nucleus, thus making it appear several times larger than it is normally. Such nuclei still have a regular, fine granular chromatin structure, and the nuclear membrane remains smooth. Nevertheless, there are often problems with distinguishing these changes from dysplastic changes (Figs. 5.1a, b, 5.2a, b). When dysplastic cells undergo cytolysis, it is often impossible to identify the epithelial layer from which they originate (Fig. 5.3). Superimposed cells may occasionally mimic the nuclei dysplastic nuclei (Fig. 5.4).
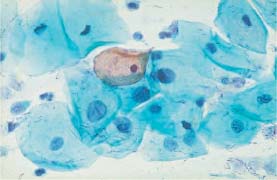
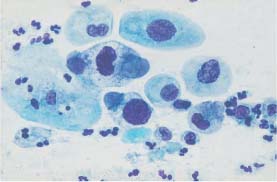
Fig. 5.2b Moderate and severe dysplasia. Hyperchromatic nuclei, irregular chromatin structure, and first signs of karyorrhexis. (DD: disturbed cell maturation.) ×630.
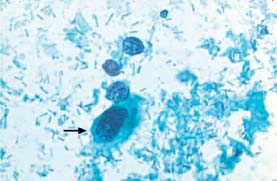
Fig. 5.3 Moderate dysplasia. Lactobacillus-induced cytolysis of a cell with abnormal nucleus and partially disintegrating cytoplasm (→). Naked nuclei of normal intermediate cells are visible nearby. (DD: severe dysplasia.) ×630.
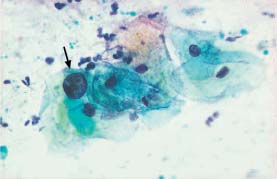
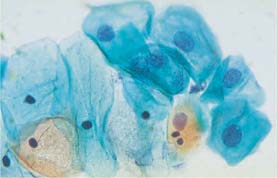
Fig. 5.5a Normal intermediate cells. Distinct nuclear swelling due to degeneration, but nuclear envelopes are still smooth. Normal superficial cells with karyopyknosis are seen nearby. (DD: moderate dysplasia.) ×630.
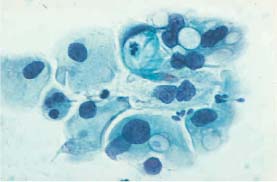
Fig. 5.5b Moderate metaplastic dysplasia Vacuolation of the cytoplasm and abnormal nuclei with first signs of degeneration. (DD: glandular dysplasia.) ×400.
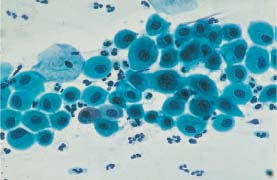
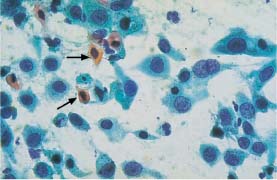
Fig. 5.6b Large-cell squamous carcinoma of the cervix. The dissociated polymorphic cells show partly still intact, partly degenerated nuclei and partly keratinized cytoplasm (→). The cells vary in size, and the cytoplasmic borders are often ill-defined. (DD: severe dysplasia.) ×630.
Cell degeneration may also cause swelling of the nuclei. These nuclei lose their chromatin structure and show reduced staining, i. e., they become hypochromatic, a feature that differentiates them from those of intact dysplastic cells (Fig. 5.5a, b).
Atrophic postpartal cells sometimes show intense cytoplasmic staining, enlarged and hyperchromatic nuclei, and a shift in the nucleocytoplasmic ratio. The resulting cell image resembles that of severe dysplasia (Fig. 5.6a, b).
Parabasal cells that show hyaline degeneration and thus resemble small horn cells are often observed in connection with atrophic vaginitis, although their round shape suggests that they are benign (Fig. 5.7a.) Polymorphism of horn cells is more pronounced with carcinoma (Fig. 5.7b.) Atrophic parabasal cells are sometimes long and spindle-shaped and resemble cells of highly differentiated keratinizing squamous carcinoma (Fig. 5.8a, b).
In old age, the smears of atrophic cells occasionally show much enlarged, and sometimes keratinized, single cells; this may be accompanied by nuclear enlargement and simultaneous degeneration. Obviously, these changes represent sporadic disturbances in cell maturation, since they are no longer detected after estrogen replacement therapy. Nevertheless, these cells are frequently confused with cells derived from dysplasia or carcinoma (Figs. 5.9a, b–5.12a, b; see also Fig. 3.18, p. 62).
Atrophic cells present in smears taken after vaginal estrogen application may, in rare cases, exhibit accelerated maturation leading to miniature superficial cells (see Fig. 3.19, p. 63), thus creating an image resembling that of dyskeratocytes or mild dysplasia.
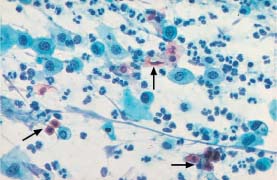
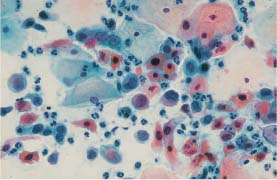
Fig. 5.7b Invasive squamous carcinoma of the cervix. The dissociated polymorphic cells have predominantly condensed, hyperchromatic nuclei. The cytoplasm shows signs of keratinization. Normal superficial cells are visible at the top. (DD: inflammation.) ×400.
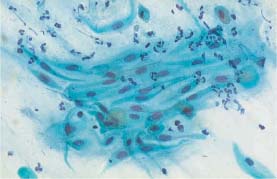
Fig. 5.8a Atrophic spindle-shaped parabasal cells in a loose, polarized cluster, exhibiting isomorphic nuclei with regular chromatin structure. (DD: keratinizing squamous carcinoma.) ×400.
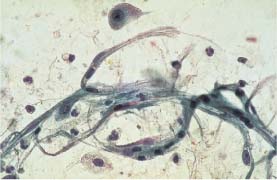
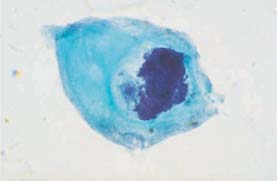
Fig. 5.9a Disturbed maturation of a parabasal cell associated with atrophy. The much enlarged, hyperchromatic nucleus shows karyorrhexis. (DD: severe dysplasia.) ×1250.
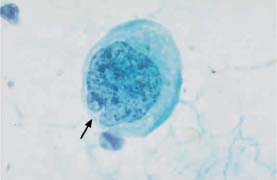
Fig. 5.9b An abnormal squamous cell associated with cervical carcinoma. The much enlarged, irregularly structured nucleus shows a cytoplasmic invagination (→). (DD: disturbed cell maturation.) ×1250.
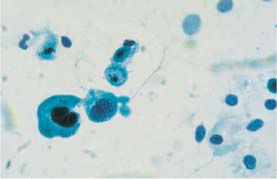
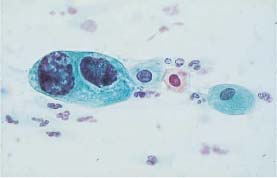
Fig. 5.10b Moderate and severe dysplasia. Binuclear cell with hyperchromatic nuclei and signs of degeneration in the form of karyorrhexis. (DD: disturbed cell maturation.) ×630.
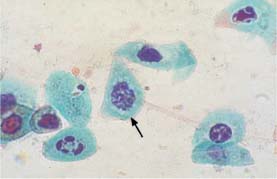
Fig. 5.11a Degenerated nuclei associated with atrophy. The nucleus in the center shows pronounced karyorrhexis and resembles a mitotic figure (→). (DD: severe dysplasia.) ×1250.
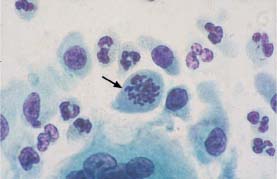
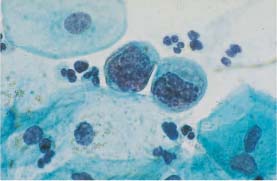
Fig. 5.12a Karyorrhexis with remnants of coarse chromatin associated with severe dysplasia. Normal intermediate cells are visible nearby. (DD: degenerated basal cells.) ×1000.
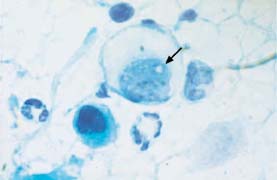
Fig. 5.12b Karyorrhexis associated with squamous carcinoma of the cervix. The nucleus in the center shows signs of disintegration resembling a hole (→). (DD: degenerated parabasal cells.) ×1250.
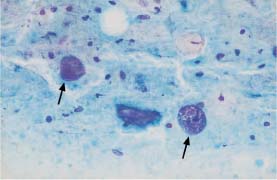
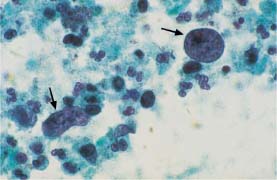
Fig. 5.13b Abnormal naked nuclei associated with uterine corpus carcinoma. Hyperchromatic nuclei with coarse chromatin structure (→)(DD: blue blobs.) ×1000.
Areas of condensed mucus (“blue blobs”) observed on top of parabasal cells during atrophy are easily confused with naked nuclei of adenocarcinoma or squamous carcinoma (Figs. 5.13a, b, 5.14a, b).
Degenerating cells with vacuoles may strongly resemble koilocytes or cells with vacuoles due to chlamydial infection (Figs. 5.15a, b, 5.16a, b).
Technical Factors
Technical damage to the cell preparation may also give rise to misinterpretations. Fixation errors and staining errors may cause pseudoeosinophilia and nuclear swelling, thus mimicking HPV infection or even mild dysplasia. Dehydration artifacts sometimes cause structural changes to the cytoplasm, and these resemble indirect signs of HPV infection and, in particular, the “cracked cytoplasm” phenomenon (Fig. 5.17a, b).
Improper smear techniques cause squeezing of the cells and lead to longitudinal extension of cells and nuclei, thus making the cells look like dysplastic spindle cells (Fig. 5.18a, b).
Occasionally, there is also retrograde transmission of horn cells from the vulva into the vagina (Fig. 5.18c).
Even years or decades after irradiation of the genital region, smears show actinic cell changes (gigantism or bizarre cytoplasmic processes), degenerative or inflammatory cells in the background, pseudoeosinophilia, amphophilia, and phagocytosis. Although the nuclei are mostly degenerate in spite of their enlargement, problems with differentiating radiation effects from dysplastic or even carcinomatous processes may arise (Fig. 5.19a, b).
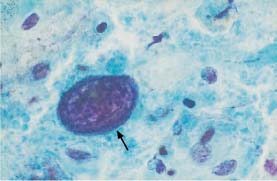
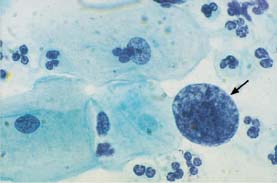
Fig. 5.14b An abnormal naked nucleus associated with squamous carcinoma of the cervix. Hyperchromatic nucleus with irregular, coarse chromatin structure (→). Also shown are normal intermediate cells. (DD: reactive enlargement of endocervical nucleus.) ×1000.
Smears taken during or after cytostatic chemotherapy frequently show enlarged, hyperchromatic, and condensed nuclei, thus resembling those of dysplastic lesions (Figs. 5.20a, b, 5.21a, b).
Macrocytosis resulting from folic acid deficiency usually involves proportional enlargement of the nucleus (Fig. 5.22a), but it may be confused with cell changes induced by radiation (Fig. 5.22b) or HPV infection.
Inflammation
The most common error is the classification of inflammatory reactions of the squamous epithelium as dysplastic processes, as they may be associated with considerable nuclear changes, particularly hyperchromatism and coarse chromatin structure (Figs. 5.23, 5.24a, b). In case of doubt, the following signs indicate benign rather than malignant changes:
- Perinuclear halos
- Regular chromatin structure
- Hyperchromatic and smooth nuclear envelopes
- Presence of chromocenters or nucleoli
- Diffuse changes of the overall cell image
However, severe granular inflammation may lead to such polymorphic cell images that differentiation from squamous carcinoma is no longer possible. On the other hand, it may also happen that a malignant process becomes masked by concurrent inflammation (Fig. 5.25a, b).
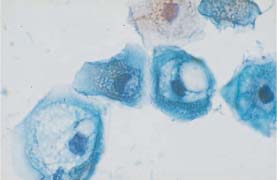
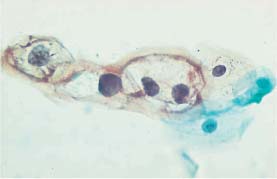
Fig. 5.15b Koilocytosis associated with mild dysplasia. The nuclei show signs of degeneration. Normal superficial cells are visible on the right. (DD: cytoplasmic degeneration.) ×400.
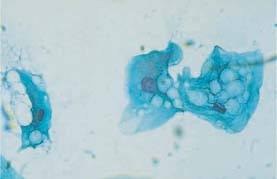
Fig. 5.16a Vacuolated cytoplasmic degeneration of intermediate cells with condensed nuclei. (DD: koilocytosis.) ×630.
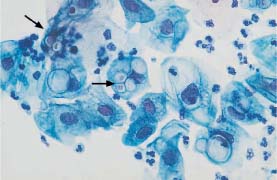
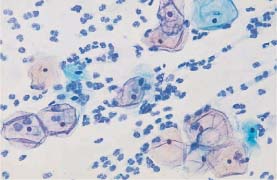
Fig. 5.17a Cytoplasmic degeneration. Thickening of the cell borders, translucency of the central cytoplasm, and pseudoeosinophilia. (DD: cracked cytoplasm associated with HPV infection.) ×320.
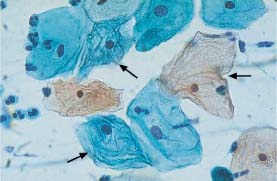
Fig. 5.17b Cracked cytoplasm of superficial cells. Intracytoplasmic ridges associated with suspected HPV infection (→) Some normal superficial cells are also present. (DD: cytoplasmic degeneration.) ×630.

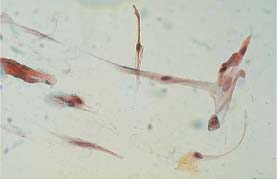
Fig. 5.18b Abnormal spindle cells associated with mild vulvar dysplasia. The nuclei are slightly enlarged and degenerated. (DD: surface effect.) ×400.
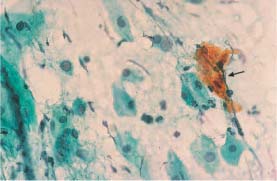
Fig. 5.18c Dyskeratotic cell cluster (→) associated with retrograde transmission ofvulvar carcinoma cells into the atrophic cervical smear. Intermediate cells and parabasal cells are visible in the background. ×400.
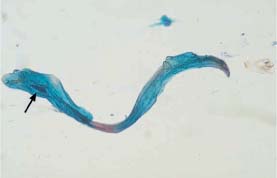
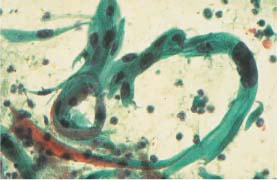
Fig. 5.19b Macrocytotic abnormal spindle cells associated with keratinizing squamous carcinoma of the cervix. The nuclei are much enlarged, hyperchromatic, and polymorphic. (DD: cell image after radiotherapy.) ×250.
An inflammation-induced tendency to keratinize is often difficult to distinguish from HPV-induced dyskeratosis, keratinizing squamous carcinoma, or adenoacanthoma (Figs. 5.26a, b–5.28a, b).
Trichomonads may look exactly like degenerating denucleated parabasal cells (Fig. 5.29a, b).
Vacuoles caused by Chlamydia occasionally show similarities to dysplastic cell-in-cell configurations (Fig. 5.30a, b).
Herpes simplex infection causes numerous cellular phenomena that may lead to misinterpretation. The size and hyperchromatism of nuclei may reach proportions similar to those in carcinomas or other malignancies (Figs. 5.31a, b, 5.32a, b), and some virus-induced giant cells resemble postradiation cells (Fig. 5.33a, b).
In the context of inflammatory processes, macrophages that phagocytose leukocytes may look very much like cells of highly differentiated adenocarcinoma (Figs. 5.34a, b, 5.35a, b).
In addition, it can sometimes be problematic to differentiate histiocytic giant cells from malignant processes (Figs. 5.36a, b, 5.37a, b).
Certain nonepithelial cells that appear during chronic inflammation, such as fibrocytes, fibroblasts, or smooth muscle cells, resemble abnormal squamous epithelial cells because of their spindle-shaped structure (Figs. 5.38a, b–5.42a, b).
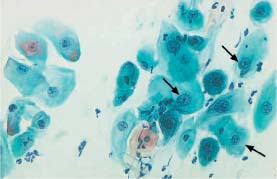
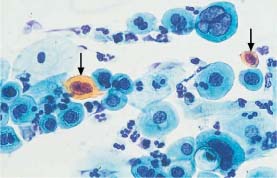
Fig. 5.20b Severe dysplasia. The nuclei are enlarged, hyperchromatic, and their chromatin structure is irregular. Individual cells are polynuclear and show signs of keratinization (→) (DD: postradiation dysplasia.) ×630.
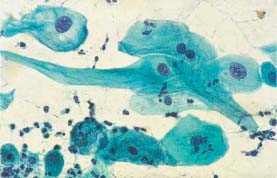
Fig. 5.21a Cell image indicating folic acid deficiency. The enlarged cells show cytoplasmic vacuolation and proportional nuclear enlargement, and some are binuclear. (DD: cell image after radiotherapy.) ×400.
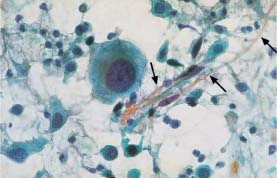

Fig. 5.22a Cell image indicating folic acid deficiency. The cells are enlarged and show proportional nuclear enlargement. (DD: cell image after radiotherapy.) ×630.
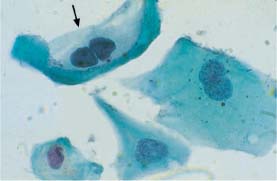
Fig. 5.22b Postradiation dysplasia. Radiation effects include cell enlargement, vacuolation of the cytoplasm, and binuclear cells. Individual cells show cytoplasmic clearing resembling that of koilocytes (→) The abnormal changes include enlarged, hyperchromatic, and polymorphic nuclei. (DD: benign radiation effects.) ×1000.
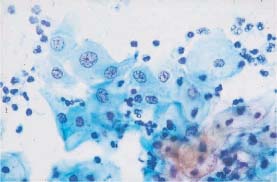
Fig. 5.24a Acute inflammation. Intermediate cells with enlarged, hyperchromatic nuclei and coarse chromatin structure. The background of the preparation indicates inflammation. (DD: moderate dysplasia.) ×500.
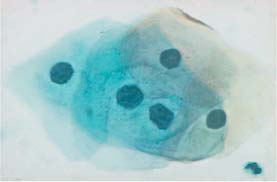
Fig. 5.24b Mild dysplasia. Enlarged and hyperchromatic nuclei with irregular chromatin structure and first signs of nuclear condensation. (DD: inflammatory reaction.) ×630.
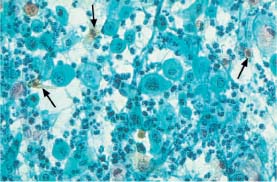
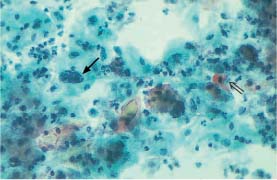
Fig. 5.25b Invasive squamous carcinoma of the cervix. Polymorphic dysplastic cells. An enlarged, hyperchromatic nucleus is seen on the left (→) and an abnormal keratinizing cell on the right (⇒). The background of the preparation indicates severe inflammation. (DD: inflammation.) ×250.
Epithelial regeneration may be associated with considerable nuclear enlargement and macronucleolus formation. This creates such a polymorphic character that confusion with poorly differentiated malignancies is possible (Figs. 5.43a, b–5.46a, b).
The surface effect caused by regeneration usually produces spindle-shaped horn cells (parakeratosis), the nuclei of which are not enlarged, unlike those seen in dysplasia and carcinoma (Figs. 5.47a, b, 5.48a, b). The cells are sometimes arranged like layers of an onion, thus forming keratin pearls. This makes it difficult to distinguish them from cells changed by HPV or from keratinized squamous carcinoma cells (Fig. 5.49a, b).
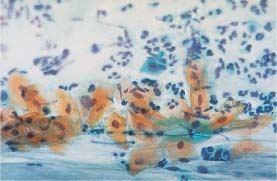
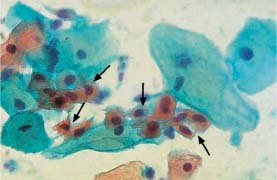
Fig. 5.26b Dyskeratocytes associated with HPV infection. Small keratinized cells with enlarged, hyperchromatic, and condensed nuclei (→). Normal superficial cells are visible nearby. (DD: surface effect.) ×630.
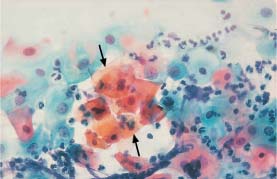
Fig. 5.27a Acute inflammation. Pseudoeosinophilic cells with signs of keratinization, especially in the center (→). The cell borders are partly blurred, and the nuclei are enlarged and hyperchromatic, while some are degenerated. (DD: mild keratinizing dysplasia.) ×400.
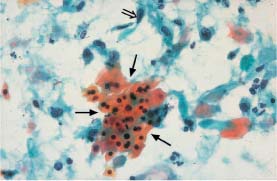
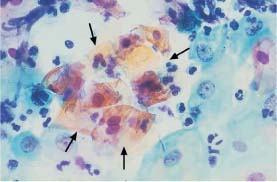
Fig. 5.28a Acute inflammation. The pseudoeosinophilic cells in the center of the image have blurred cell borders (→) The nuclei of the surrounding intermediate cells are enlarged and partly exhibit nucleoli. (DD: moderate dysplasia.) ×630.
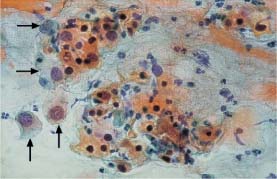
Fig. 5.28b Adenocarcinoma of the endocervix with a squamous epithelial component. In addition to abnormal glandular cells (→), there are keratinizing squamous cells with hyperchromatic, condensed nuclei. (DD: severe keratinizing dysplasia.) ×400.
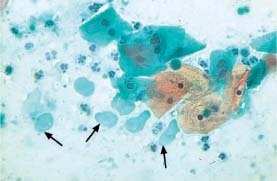
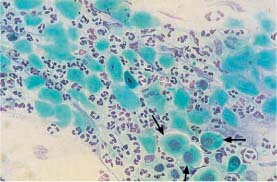
Fig. 5.29b Degenerated parabasal cells associated with atrophy. The nuclei show severe signs of degeneration and even signs of complete lysis. The background of the preparation indicates inflammation. A few preserved parabasal cells are visible on the lower right (→) (DD: trichomonadal vaginitis.) ×400.
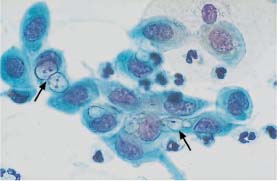
Fig. 5.30a Chlamydial infection. Immature metaplastic cells containing poorly defined vacuoles of various size and inclusion bodies (→) The nuclei are active and enlarged. (DD: severe dysplasia.) ×1000.
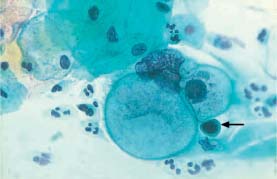
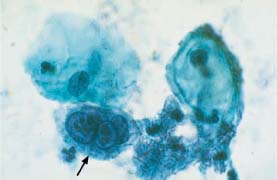
Fig. 5.31a Herpes simplex infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



