DEFINITION OF THE COMPLAINT
Diarrhea is one of the most common conditions for which patients seek medical care. It is a condition that continues to be associated with significant morbidity and mortality worldwide, despite medical advances. It is characterized by an increase in the frequency, volume, or liquid content of stool as compared to any given individual’s usual pattern.
Diarrhea may also be further characterized by the duration of the symptoms, with acute episodes of diarrhea generally resolving within 2 weeks, while chronic diarrhea generally lasts longer than 2 weeks. Another important distinction in the type of diarrhea is based on whether it is secretory or osmotic in nature. Agents that disrupt the normal absorption of intestinal luminal fluid at the cellular level generally cause a profuse and voluminous secretory diarrhea that continues regardless of the patient’s oral intake. Osmotic diarrhea, however, is the result of poorly absorbed substances that draw fluid into the intestinal lumen. This type of diarrhea tends to improve with fasting on the part of the patient.
The most common causes of diarrhea are infectious, with viral etiologies occurring more frequently than bacterial. The differential diagnosis of diarrhea, however, is quite extensive and includes some rare causes. Many cases of diarrhea occur in children who are otherwise well appearing, while some cases of diarrhea present in children who are ill appearing, due to either poor nutrition, hydration, or other systemic reasons.
COMPLAINT BY CAUSE AND FREQUENCY
There are myriad causes of diarrhea that can be stratified by age (Table 17-1) or diagnostic category (Table 17-2).
TABLE 17-1. Causes of diarrhea by age.
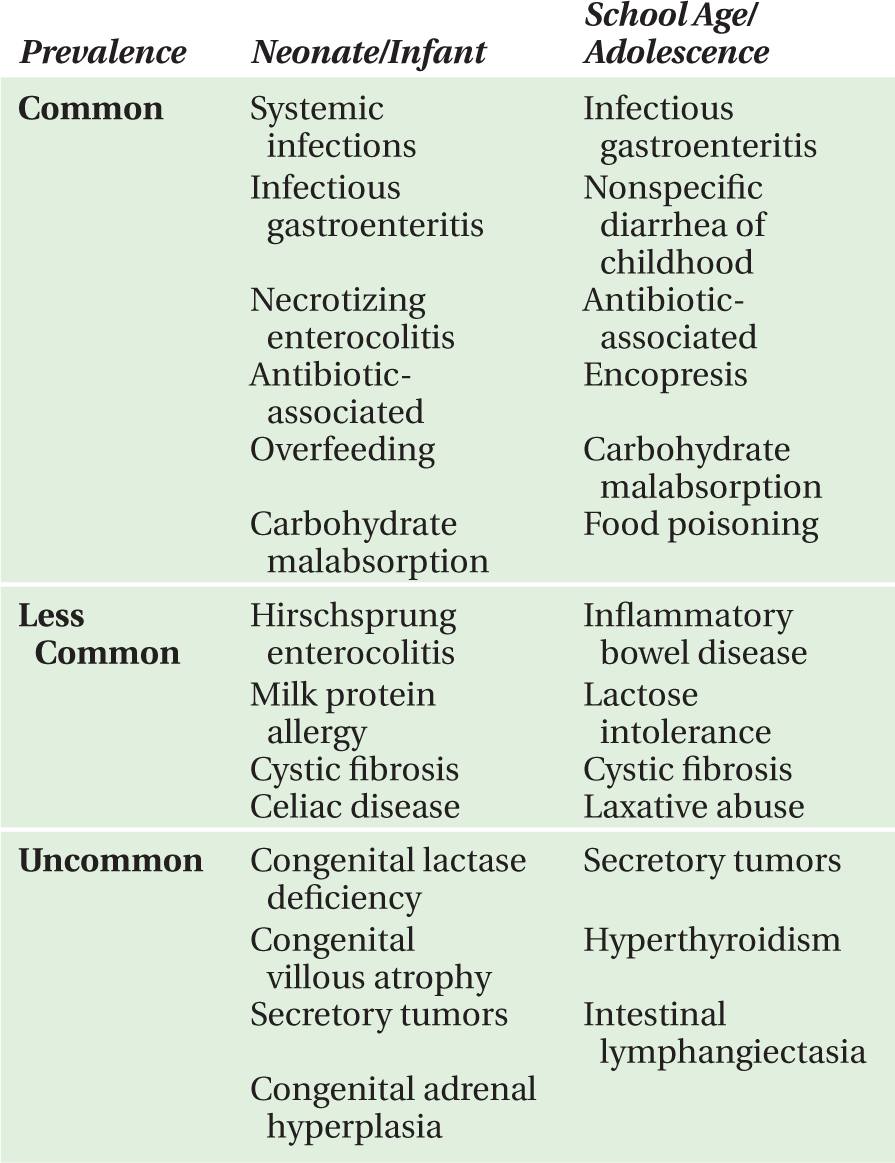
TABLE 17-2. Causes of diarrhea by diagnostic category.

CLARIFYING QUESTIONS
A thorough history can provide clues to facilitate an accurate diagnosis in the child who presents with diarrhea. Consideration of the age and appearance of the patient, the length and course of the illness, and associated clinical features provides a useful framework for creating a differential diagnosis. The following questions may help provide clues to the diagnosis:
• How long has the diarrhea lasted?
—Diarrhea that has lasted less than 2 weeks is acute diarrhea, rather than chronic. Acute diarrhea is more likely to be infectious (viral or bacterial) in etiology. Chronic diarrhea raises the concern over other diagnoses such as malabsorptive conditions (cystic fibrosis, celiac disease), although infectious (parasitic) and postinfectious (postinfectious carbohydrate malabsorption) causes are still possible.
• Is there any blood or mucus in the stool?
—In the acute setting, blood or mucus in the stool increases the possibility of an enteroinvasive agent (enteroinvasive Escherichia coli, Salmonella spp. or Shigella spp.). In the chronic setting, inflammatory bowel disease should be considered. In a systemically ill-appearing child, hemolytic uremic syndrome must be considered.
• Is there abdominal pain or cramping? Tenesmus?
—Acute infectious gastroenteritis can present with abdominal cramping, while a chronic history of cramping or tenesmus raises the concern for inflammatory bowel disease.
• Is there any vomiting?
—Vomiting may be associated with acute infectious gastroenteritis. However, if bilious vomiting is noted, especially in a neonate or an infant, an anatomic condition (malrotation, incarcerated hernia) must be considered.
• Is there a fever?
—Presence of a fever acutely may indicate either an enteroinvasive infectious agent or systemic illness (pneumonia) with an associated nonspecific diarrhea. In a toxic-appearing child, sepsis and toxic shock syndrome must be considered. In a patient with a chronic history of diarrhea with acute exacerbations associated with fever, inflammatory bowel disease is a distinct possibility.
• Does the patient appear systemically ill?
—In acute diarrhea, a systemically ill-appearing child should raise the concern for sepsis (Salmonella spp., E. coli, especially in a neonate or infant). If oliguria is also present, hemolytic uremic syndrome must be considered, in addition to simple dehydration associated with diarrheal losses. In patients who have a history of chronic diarrhea and failure to thrive, superimposed episodes of acute diarrhea can make them appear systemically ill, as in cases of inflammatory bowel disease, celiac disease, or cystic fibrosis.
• Is there failure to thrive?
—A chronic history of diarrhea associated with failure to thrive raises the concern for malabsorptive conditions such as cystic fibrosis and celiac disease. Neuroendocrine tumors that cause a secretory diarrhea may present with significant weight loss. Inflammatory bowel disease also commonly presents with linear growth arrest in addition to poor weight gain.
• Are there ill contacts with diarrhea?
—Close contacts with similar symptoms may indicate an outbreak with a common source of contamination (e.g., daycare, family reunion, restaurant), whether toxin-associated food poisoning, or fecal-oral contamination.
• Is there any unusual food exposure?
—In particular, undercooked foods, specifically beef, are of concern as a source for E. coli O157:H7 resulting in hemolytic uremic syndrome (HUS). Improperly stored food is another potential source for food poisoning. New foods may not be tolerated well and be the source of transient diarrhea or may cause bloody diarrhea, as in the case of milkprotein allergy in infants.
• Any recent history of travel?
—Foreign travel increases the concern over travelers’ diarrhea, often due to unfamiliar strains of E. coli, or unusual organisms, such as Entamoeba histolytica, as a cause of chronic diarrhea. Other parasites such as Giardia lamblia and agents such as hepatitis A may also be acquired during travel.
• What is the water source?
—Untreated or contaminated water sources can harbor Giardia lamblia or Cryptosporidium. Cases of E. coli O157:H7 transmission have also been known to occur with exposure in water sources such as swimming pools or lakes.
• Are there any pets? Any exposure to animals?
—Pets such as lizards, turtles, and iguanas may harbor Salmonella, which can then cause diarrhea in children who play with them. Farm animals and petting zoos are also potential sources for E. coli O157:H7 and epidemic cases of hemolytic uremic syndrome.
• Is there a history of recent antibiotic use?
—Antibiotic-associated diarrhea, including Clostridium difficile colitis, may occur.
• Is there any significant medical history?
—Failure to thrive is of particular concern with either superimposed acute or chronic diarrhea. Former premature infants who had surgical necrotizing enterocolitis may have subsequent chronic diarrhea due to short bowel syndrome. Other conditions may also have diarrhea associated (human immunodeficiency virus infection and other immune compromising conditions) as well as endocrinologic disorders (e.g., hyperthyroidism).
• Is there a significant family history?
—Patients with inflammatory bowel disease may present with family members with similar symptoms. Cystic fibrosis and celiac disease have traditionally been associated with Northern European ancestry, although patients of other ethnicities can also carry these diagnoses.
• Is the diarrhea worse with oral intake? Is it improved with fasting?
—This question will help to differentiate osmotic diarrhea, which characterizes most cases of diarrhea, from secretory diarrhea, which is much less common and often is associated with otherwise occult oncologic conditions.
• Is there a rash?
—A petechial, purpuric rash would be indicative of Henoch-Schönlein purpura, although, in an ill-appearing child, sepsis would also have to be considered. Other rashes, such as dermatitis herpetiformis, can be seen in chronic conditions, such as celiac disease. Rashes may also develop due to nutritional deficiencies.
• Is the weight loss intentional?
—Teenagers who are overly concerned with body image may be using laxatives to lose weight.
Two-Month-Old Boy
HISTORY OF PRESENT ILLNESS
The patient is a 2-month-old boy who presents with vomiting and diarrhea. The patient had been recently discharged from the hospital 3 days ago. During that previous hospitalization, he had been diagnosed with gastroesophageal reflux by pH probe and upper gastrointestinal series. He had been discharged to home on ranitidine and had been doing well until the evening prior to presentation when he developed vomiting and diarrhea. He had 12 episodes of nonbloody, nonbilious vomiting with eight episodes of loose stools. There was no fever or associated upper respiratory symptoms. He had normal urine output. His mother reports that he was more fussy than usual and she noted a lump in his groin on the day of presentation to the hospital.
MEDICAL HISTORY
The patient was a full-term baby with an uncomplicated pregnancy, labor, and delivery history. He was hospitalized only once, diagnosed with gastroesophageal reflux, and placed on ranitidine.
PHYSICAL EXAMINATION
T 36.9°C; RR 32/min; HR 136 bpm; BP 100/54 mmHg
Weight 5th percentile
On examination, the infant was alert and in no acute distress. His head, neck, cardiac, and respiratory examination were unremarkable. He was well hydrated with a nontender and non-distended, soft abdomen. There was no hepatosplenomegaly or abdominal masses. He had normal male genitalia, with bilaterally descended testicles. A tender, firm, and erythematous mass measuring 5 cm × 3 cm was palpable in the right inguinal region.
DIAGNOSTIC STUDIES
The complete blood count revealed a WBC count of 10 100 cells/mm3 (11% segmented neutrophils, 76% lymphocytes), a hemoglobin of 10.8 g/dL with a mean corpuscular volume of 87 fL and a platelet count of 387 000 mm3. Serum electrolytes, blood urea nitrogen, and creatinine were normal.
COURSE OF ILLNESS
An abdominal radiograph obtained on his previous admission suggested a cause for the current complaint (Figure 17-1). A surgical consultation was requested.

FIGURE 17-1. Abdominal radiograph.
DISCUSSION CASE 17-1
DIFFERENTIAL DIAGNOSIS
In this case, diarrhea was associated with vomiting and a critical physical finding, that of an inguinal mass. This essential finding directs the differential diagnosis toward causes of inguinal or scrotal swelling. An important distinction to make is between a painful or painless mass. A hydrocele is a common entity that causes painless inguinal or scrotal swelling. It is primarily differentiated from an inguinal hernia by the ability to palpate above the mass, revealing discontinuity between the mass and the inguinal canal. The mass, as a result, does not change in size with straining or crying. In addition, a hydrocele is not reducible and usually transilluminates, although the ability to transilluminate the mass does not exclude the possibility of an incarcerated hernia.
Another cause of a painful scrotal mass is testicular torsion. There often is no history of a prior scrotal mass, and in fact may be associated with a history of undescended testis. This mass is very tender and does not extend into the inguinal canal.
Torsion of the appendix testis results in a painful scrotal mass that may present as a tender blue nodule on the upper pole of the testis which, itself, is not tender. Inguinal lymphadenopathy may be tender or painless but the key to diagnosis is the lateral and inferior location of these nodes in relation to the inguinal canal. Signs of infection in the area of lymphatic drainage are also important in making this diagnosis. An inguinal hernia is usually characterized by a painless swelling in the inguinal area often increasing in size with crying or straining. Incarceration of the hernia results in extreme pain and signs of bowel obstruction. If strangulation occurs, bloody diarrhea may occur.
DIAGNOSIS
A thorough history and physical examination are the keys to this diagnosis. In this case, the painful nature and inguinal location of this mass are the essential findings. Abdominal radiograph from the previous admission revealed a right inguinal hernia (Figure 17-1, arrow) that is now incarcerated. The diagnosis is incarcerated inguinal hernia. The hernia was reduced in the emergency department by pediatric surgical staff. No hernia was noted on the left side on physical examination. The patient was admitted for intravenous fluids and observation to allow the bowel edema from the incarceration to resolve. The patient manifested no signs or symptoms of bowel necrosis during 2 days in the hospital after which he was taken to the operating room. Intraoperatively, bilateral inguinal hernias were found and repaired without any complications.
INCIDENCE AND EPIDEMIOLOGY OF INGUINAL HERNIA
The incidence of inguinal hernia is estimated to be anywhere from 1% to 5%, which is approximately 10-20 per 1000 live births. The incidence in premature infants is significantly higher, approaching 30%. The ratio of boys to girls is 6:1. In boys, the right side is more frequently involved than the left, presumably due to the embryologic origin of inguinal hernias through a patent processus vaginalis and the fact that the right testis descends later during gestation than the left. In both boys and girls, 60% of inguinal hernias occur on the right, 30% on the left, and 10% bilaterally. Inguinal hernias are usually diagnosed during the first year of life, most frequently in the first month of life. There is often a family history of inguinal hernia. Undescended testes may also be associated with inguinal hernias. Other conditions associated with inguinal hernias include Ehlers-Danlos syndrome, cystic fibrosis, congenital cytomegalovirus infection, and testicular feminization. There is no apparent ethnic or racial predisposition to inguinal hernia. Incarcerated inguinal hernias occur most frequently in those younger than 6 months of age, are less common after 2 years of age, and are rare after 5 years of age.
CLINICAL PRESENTATION OF INGUINAL HERNIA
An inguinal hernia usually presents as an asymptomatic swelling in the scrotal or labial area that increases in size with any increase in intra-abdominal pressure, as occurs with crying or straining. Reducible hernias disappear spontaneously or with minimal pressure. An incarcerated hernia develops when a loop of bowel becomes trapped and is accompanied by severe pain and signs of bowel obstruction, such as bilious emesis. Strangulation of the herniated loop of bowel occurs when the blood supply to the bowel is compromised and may develop within 2 hours of incarceration. Urgent medical attention is necessary in cases of incarceration and emergency surgical intervention may be necessary in cases of strangulation.
DIAGNOSTIC APPROACH
The key to diagnosis of inguinal hernia lies in the index of suspicion in the appropriate historical context, which is then confirmed by physical examination. In distinguishing an incarcerated hernia, an awareness of the other important entities in the differential diagnosis is important. The diagnosis itself is primarily founded on the history and physical examination as well as a thorough knowledge of the disease process.
Abdominal radiograph. An abdominal radiograph may show signs of bowel obstruction and may serve as an adjunctive supportive piece of evidence in making the diagnosis.
TREATMENT
In cases of incarcerated hernia, time is of the essence. Compromised blood flow to the affected loop of bowel may result in strangulation and bowel necrosis within 2 hours, hence medical intervention is necessary. Attempt at reduction of the incarcerated hernia by experienced pediatric surgical staff is optimal. A gentle attempt at reduction using pressure on the scrotum with simultaneous counterpressure above the external inguinal ring is indicated, but should never be forcefully done. Intravenous hydration and nasogastric tube decompression, in anticipation of definitive surgical management, is also indicated. Immediate surgical correction is necessary if the incarcerated hernia is not reducible. If the incarcerated loop of bowel is reduced, surgery may be postponed for 12-36 h to allow the bowel edema to resolve.
Elective repair of an asymptomatic inguinal hernia should be performed as soon as possible after diagnosis to avoid complications, such as incarceration. All inguinal hernias require surgical correction as they do not resolve spontaneously. In boys, undescended testes may be associated with inguinal hernia and require orchiopexy. There is still ongoing debate as to the importance of surgical exploration of the contralateral side in search of an occult inguinal hernia not detected by physical examination, as did occur with the patient in this case. This decision is left to the individual surgeon, but contralateral exploration is performed in the majority of cases.
SUGGESTED READINGS
1. Clarke S. Pediatric inguinal hernia and hydrocele: an evidence based review in the era of minimal access surgery. J Laparoendosc Adv Surg Tech A. 2010;20(3):30-39.
2. Brandt ML. Pediatric hernias. Surg Clin North Am. 2008;88(1):27-43, vii-viii.
3. Katz D. Evaluation and management of inguinal and umbilical hernias. Pediatr Ann. 2001;30:729-735.
4. Kapur P, Caty M, Glick P. Pediatric hernias and hydroceles. Pediatr Clin North Am. 1998;45:773-789.
5. Irish M, Pearl R, Caty M, Glick P. The approach to common abdominal diagnoses in infants and children. Pediatr Clin North Am. 1998;45:729-772.
CASE 17-2
Two-Year-Old Boy
HISTORY OF PRESENT ILLNESS
The patient is a 2-year-old boy, who had been previously well until 3 days prior to presentation to the hospital with watery diarrhea and decreased appetite. The next day, he also developed vomiting at which point he was seen by his primary physician who treated him with trimethobenzamide hydrochloride (Tigan) suppositories which provided no relief. The symptoms progressed to 20 episodes of diarrhea, now with blood and mucus and abdominal cramping on the day of presentation. The patient was admitted to an outside hospital for presumed bacterial gastroenteritis. There were no known ill contacts, no known ingestion of undercooked foods, no recent travel, and no recent exposure to antibiotics.
MEDICAL HISTORY
The boy’s history was significant only for one hospital admission for an asthma exacerbation. There was no surgical history, no medications. His mother has a history of irritable bowel syndrome.
PHYSICAL EXAMINATION
T 37.5°C; RR 30/min; HR 150 bpm; BP 105/50 mmHg
Weight 50th percentile
The patient was alert, but quiet and ill appearing. His eyes were mildly sunken. He had dry mucous membranes. There was no lymphadenopathy. The remainder of his head and neck examination was unremarkable. His cardiac examination revealed tachycardia, but no murmur or gallop. There was tachypnea, but no rales or wheezing. His neurologic examination was nonfocal. His skin turgor was diminished.
DIAGNOSTIC STUDIES
Complete blood count revealed a WBC count of 16 600 cells/mm3 with a differential of 62% segmented neutrophils, 24% band forms, 10% lymphocytes, 3% monocytes, and 1% atypical lymphocytes. Hemoglobin was 14.2 g/dL and platelet count was 381 000 cells/mm3. White blood cells were present on Gram stain of the stool. Routine bacterial and viral stool cultures were sent which eventually returned negative. Serum electrolytes were significant for a chloride of 100 mmol/L and a bicarbonate of 19 mmol/L. Blood urea nitrogen was 73 mg/dL and creatinine was 1.2 mg/dL.
COURSE OF ILLNESS
The patient was admitted to the hospital for intravenous fluid rehydration and given nothing by mouth after failing of repeated oral clear liquid challenges while continuing to have diarrhea. A Foley catheter was placed to monitor urine output (Figure 17-2). On the second day of hospitalization the patient developed a fever to 39°C with increased abdominal pain, particularly in the periumbilical region and right lower quadrant. His abdominal examination now revealed hypoactive bowel sounds and an abdominal radiograph demonstrated ileus. An abdominal ultrasound showed a small amount of ascites. Ampicillin, gentamicin, and clindamycin were started intravenously. A sigmoidoscopy was performed to 50 cm, demonstrating a normal appearing colon. A biopsy was obtained which showed signs of chronic inflammation. Later that day, the patient was taken for an exploratory laparotomy due to worsening abdominal pain and signs of an acute abdomen. Surgery revealed a leathery thickening of the descending colon, possibly consistent with chronic inflammation, without involvement of the transverse colon or distal sigmoid. A large amount (500 mL) of clear yellow ascitic fluid was also removed. A central venous catheter was placed, given the anticipated need for prolonged intravenous fluids and medications.
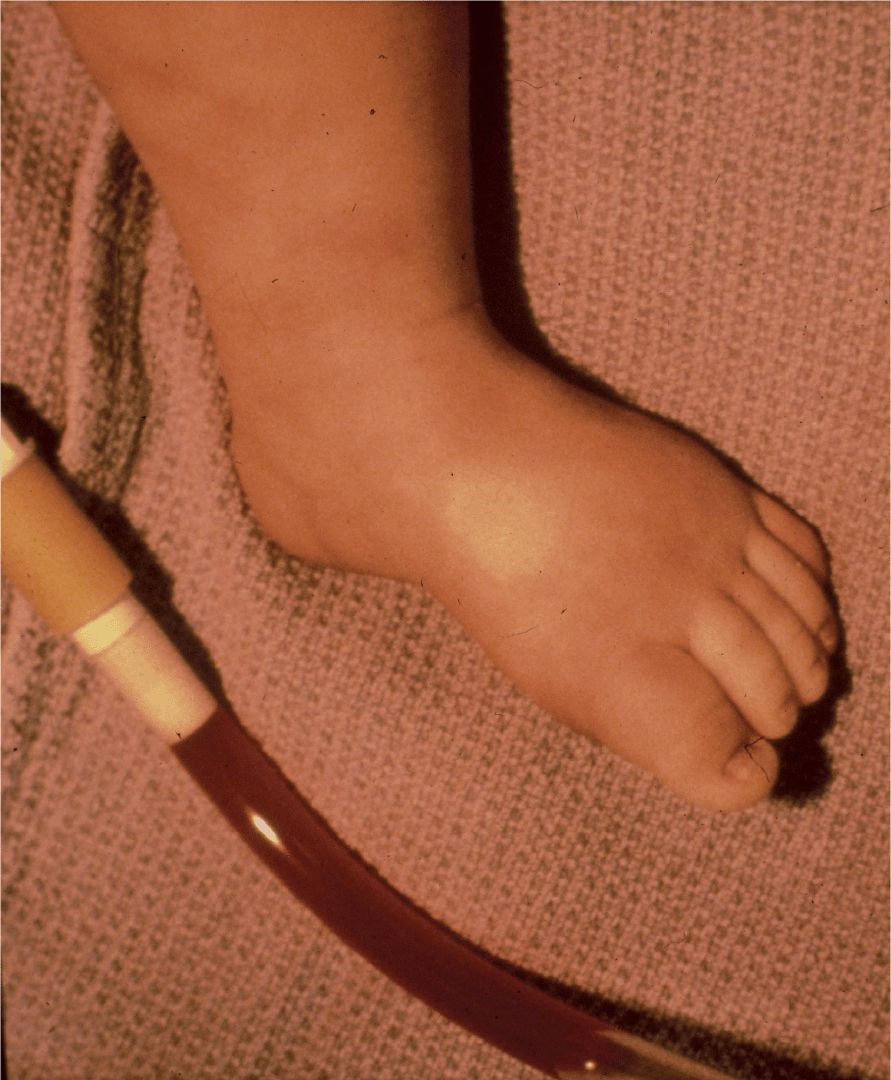
FIGURE 17-2. Photo of the patient’s leg (note the poor perfusion) that also includes a portion of the urinary catheter drainage.
DISCUSSION CASE 17-2
DIFFERENTIAL DIAGNOSIS
The key to this diagnosis is having the appropriate level of suspicion in the right clinical context. During the initial prodrome of bloody diarrhea, any of the other bacterial causes of enteroinvasive diarrhea would be on the list of differential diagnoses. Salmonella, Shigella, and Campylobacter species would be among some of the etiologies. In fact, HUS has been associated with Shigella infection, as a result of elaboration of Shiga toxin. All of these bacterial causes would be routinely screened for in most stool cultures. It is important to remember that E. coli O157:H7 may not be routinely screened for, although it may now be the most common cause of bloody diarrhea in the United States. Clostridium difficile colitis could also present in this manner and testing for toxins A and B would be important in distinguishing this entity, as would a prior history of recent antibiotic use.
Inflammatory bowel disease could also present in this fashion, especially as an acute flare with signs of systemic toxicity and abdominal symptoms severe enough sometimes to warrant surgical exploration.
DIAGNOSIS
The Foley catheter revealed bloody urine (Figure 17-2). The patient’s lower extremity, also included in the photograph, revealed evidence of poor perfusion. Postoperatively the patient was noted to be edematous while on maintenance intravenous fluids. Repeat laboratory studies revealed a serum sodium of 133 mmol/L, chloride 114 mmol/L, bicarbonate 11 mmol/L, blood urea nitrogen 24 mg/dL, and creatinine 3.0 mg/dL. The hemoglobin decreased to 11.3 g/dL and the platelet count was 118 000 cells/mm3. The peripheral smear showed the presence of schistocytes, suggesting a hemolytic process. Liver function tests were significant for an aspartate aminotransferase of 532 U/L, alanine aminotransferase 287 U/L, lactate dehydrogenase 4290 U/L, and albumin 1.7 g/dL. The partial thromboplastin time was slightly elevated at 37.4 seconds. During the next 3 hours, the patient’s urine output stopped completely. He was given 25% albumin intravenously with intravenous fluids followed by furosemide which resulted in only 10 cc of urine output. At this point, the patient was transferred to our institution for continuous arteriovenous hemoperfusion. A stool culture sent specifically for detection of E. coli O157:H7 returned positive. This culture confirmed the diagnosis of hemolytic uremic syndrome (HUS) secondary to infection with E. coliO157:H7, otherwise known as D(+) HUS, in that it is associated with diarrhea. During his 4-week hospitalization, his blood urea nitrogen level peaked at 101 mg/dL and creatinine at 9.9 mg/dL. His hemoglobin nadir was 4.7 g/dL and platelet count nadir was 103 000/mm3. He required continuous arteriovenous hemoperfusion for most of the remainder of his hospitalization. Upon discharge, his blood urea nitrogen was 74 mg/dL and his creatinine was 5.5 mg/dL.
INCIDENCE AND EPIDEMIOLOGY OF HEMOLYTIC UREMIC SYNDROME
HUS is one of the most common causes of acute renal failure in children, and in turn, E. coli O157:H7 infection is the most common cause of HUS. As a member of the enterohemorrhagic group of E. coli, it causes a hemorrhagic colitis and elaborates a verotoxin that is similar to Shiga toxin, produced by Shigella dysenteriae type 1. In the United States, it appears to be one of the most common causes of bloody diarrhea. Cases can occur on a sporadic as well as epidemic basis, which has appeared to be increasing in the last decade. There is a seasonal pattern to infection, in that cases are more common in the summer months, although cases do sometimes occur in the winter. This may also be associated with the fact that cattle and their undercooked meat products or unpasteurized dairy products are the most significant factors in transmission and are more likely to be consumed during the summertime.
The highest attack rates occur at the extremes of the age spectrum—the very old and the very young appear to be at greatest risk. In particular, children younger than 4 years of age are at great risk of contracting E. coli O157:H7 infection and are also at greater risk for developing HUS as a result. HUS occurs in approximately 10% of patients who acquire sporadic infection with E. coli O157:H7 and appears to occur at a higher attack rate of up to 40% of patients infected in an outbreak. The mortality rate is approximately 5% and another 5% of patients suffer from severe neurologic sequelae or end-stage renal failure. Extremely poor prognostic factors include a high leukocyte count, a severe gastrointestinal prodrome, age younger than 2 years, and early onset of anuria.
CLINICAL PRESENTATION OF HEMOLYTIC UREMIC SYNDROME
Infection with E. coli O157:H7 begins as a non-bloody diarrhea that progresses to bloody diarrhea, often a few days after the onset of illness. Vomiting occurs in half of the patients and a fever in approximately one-third. Fecal leukocytes are often present. The hemorrhagic colitis is otherwise nonspecific and may appear indistinguishable clinically from many other colitides. Abdominal symptoms may become severe enough to mimic an acute abdomen, prompting surgical intervention. Complications include intussusception, perforation, or stricture. The classic triad of microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure follows the gastrointestinal prodrome. Leukocytosis develops, as can cerebral edema with seizure activity. Irritability progressing to stupor and coma may also develop. Cortical blindness and stroke may occur as well.
HUS may present in a D(–) form which is not associated with diarrhea. Cases of D(–) HUS, otherwise known as atypical HUS, have been associated with medications, familial patterns of inheritance, recurrent episodes of HUS, and bacterial infections, such as Streptococcus pneumoniae. In general, patients with D(–) HUS have a higher incidence of neurologic sequelae in addition to the renal sequelae and overall have worse outcomes than patients with D(+)HUS.
HUS is occasionally associated with S. pneumoniae. Children with pneumococcal HUS typically present with pneumonia complicated by empyema. Blood cultures are typically positive. In one multicenter case series of children with pneumococcal HUS, 4 of 37 patients have concomitant pneumococcal meningitis. Up to one-third of children with pneumococcal HUS have residual renal dysfunction, including elevated creatinine, proteinuria, and hypertension.
DIAGNOSTIC APPROACH
Clinical and laboratory findings. The key to diagnosis is recognition of the clinical syndrome in the appropriate context. An abnormal complete blood count with thrombocytopenia and anemia with schistocytes and red blood cell fragments on the peripheral smear in the context of fluid overload and oliguria or anuria with a rising serum creatinine confirm the diagnosis of this syndrome.
Microbiology. Stool culture is important to rule out other infectious causes of colitis, but specific biochemical tests are necessary to identify the specific serotype, O157:H7, and in some laboratories, may not be routinely performed and must be specifically requested.
Enzyme immunoassays. New, rapid methods to detect E. coli O157:H7 lipopolysaccharide and shiga toxin are very useful in diagnosing infection in a timely manner.
TREATMENT
Current therapy remains supportive in nature; however, prevention is essential by ensuring complete cooking of all beef products, particularly ground beef, thorough hand-washing when interacting with animals which may be carriers, and avoidance of unpasteurized dairy or other products. Patients who develop HUS require significant volume-support with careful fluid and electrolyte management. Transfusion may be necessary due to significant gastrointestinal blood loss and microangiopathic hemolytic anemia. Hypertension and renal failure must be anticipated in the clinical management and patients often require dialysis. Neurologic complications, such as seizures, may require antiepileptic therapy. There is no evidence that antibiotics are helpful and they may be potentially harmful, by causing increased release of toxin. Antimotility agents are also contraindicated because of the increased absorption of toxin.
SUGGESTED READINGS
1. Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60.
2. Zoja C, Buelli S, Morigi M. Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatr Nephrol. 2010;2(11):2231-2240.
3. Bitzan M, Schaefer F, Reymond D. Treatment of typical (enteropathic) hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36(6):594-610.
4. Malina M, Orumenina LT, Seeman T, et al. Genetics of hemolytic uremic syndromes. Presse Med. 2012;41(3Pt2): e10-e14.
5. Scheiring J, Rosales A, Zimmerhackl LB. Clinical practice: today’s understanding of haemolytic uremic syndrome. Eur J Pediatr. 2010;169(1):7-13.
6. Palerma MS, Exeni RA, Fernandez GC. Hemolytic uremic syndrome: pathogenesis and update of interventions. Expert Rev Anti Infect Ther. 2009;7(6): 697-707.
7. Copelovitch L, Kaplan BS. Streptococcus pneumoniae-associated hemolytic uremic syndrome. Pediatr Nephrol. 2008;23(11):191-196.
8. Iijima K, Kamioka I, Nosu K. Management of diarrhea-associated hemolytic uremic syndrome. Clin Exp Nephrol. 2008;12(1):16-19.
9. Banerjee R, Hersh AL, Newland J, et al. Streptococcus pneumoniae-associated hemolytic uremic syndrome among children in North America. Pediatr Infect Dis J. 2011;30:736-739.
10. Waters AM, Kerecuk L, Luk D, et al. Hemolytic uremic syndrome associated with invasive pneumococcal disease: the United Kingdom experience. J Pediatr. 2007;151:140-144.
CASE 17-3
Seventeen-Year-Old Boy
HISTORY OF PRESENT ILLNESS
The patient is a 17-year-old boy who presents with 7 months of loose nonbloody stool and 1 week of fever to 39.5°C, severe abdominal pain, and bloody diarrhea. The patient was well until 7 months ago when he began having 3-4 loose stools per day whenever he ingested food. During those 7 months, he had no fevers, abdominal pain, nausea, emesis, bloody stool, rash, or arthritis. One week prior to admission, he developed intermittent fevers to a maximum of 39.5°C, periumbilical abdominal pain with ingestion of food, and multiple episodes per day of grossly bloody stool. On admission, the patient had just completed a 10-day course of oral clarithromycin for sinusitis. He denied recent travel outside the country, ingestion of undercooked food, or sick contacts. He did admit to a 7-lb weight loss during the past 7 months that had been unintentional.
MEDICAL HISTORY
Medical history is significant for seasonal allergic rhinitis for which the patient takes loratadine (Claritin) as needed. There is no known family history of any gastrointestinal or bleeding disorders.
PHYSICAL EXAMINATION
T 37°C; RR 18/min; HR 60 bpm; BP 120/65 mmHg
Weight 55 kg, 10th percentile; Height 170 cm, 25th percentile
Physical examination revealed a lean but non-emaciated boy in no acute distress. He was anicteric with moist mucous membranes. Cardiac and respiratory examinations were normal. His abdomen was soft, nontender, and nondistended without hepatosplenomegaly or palpable masses. He had normoactive bowel sounds. On external anal examination he had no visible fissures, tags, or masses. Rectal examination revealed grossly heme-positive soft stool in the rectal vault. There were no palpable rectal masses. His skin examination revealed no rashes, petechiae, or purpura. Neurologic examination was normal.
DIAGNOSTIC STUDIES
Serum electrolytes, glucose, blood urea nitrogen, and creatinine were all normal. A complete blood count (CBC) revealed a WBC count of 23 700 cells/mm3, hemoglobin of 11.7 g/dL, and a platelet count of 302 000/mm3. Coagulation factors including PT, PTT, and INR were all normal. The erythrocyte sedimentation rate (ESR) was 5 mm/h and the C-reactive protein (CRP) was 1.5 mg/dL. A hepatic function panel was normal except for a serum albumin that was low at 2.4 g/dL and a serum alkaline phosphatase that was low at 58 U/L. Stool samples were negative for Clostridium difficile, bacterial pathogens, viral pathogens, and ova and parasites.
COURSE OF ILLNESS
The patient was initially admitted to an outside hospital where he underwent flexible sigmoidoscopy, which reportedly showed inflammation that the physicians believed to be consistent with inflammatory bowel disease. The patient was placed on mesalamine enemas as well as oral mesalamine, omeprazole, and prednisone. He had some improvement in stool frequency, consistency, and amount of fecal blood and was discharged home.
Five days after discharge, the patient presented to our hospital with recurrence of bloody diarrhea and abdominal pain. After rehydration he underwent a complete endoscopy and colonoscopy that revealed multiple polyps in the small bowel and throughout the colon (Figure 17-3). Several polyps were removed and were sent to pathology for review. Due to findings seen on pathology, a retinal examination was performed which suggested a diagnosis.

FIGURE 17-3. Colonic polyps. (Reproduced, with permission, from McQuaid KR. Gastrointestinal disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. CURRENT Medical Diagnosis & Treatment 2013. New York: McGraw-Hill; 2013. http://www.accessmedicine.com/content.aspx?aID=6395. Accessed April 25, 2013.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


