Fig. 2.1
Common locations of pediatric bone tumors. The location of a bone tumor is frequently the first information available to narrow the differential diagnosis of a bone lesion. This illustration demonstrates the common location of many pediatric bone tumors
2.1.1 Basic Radiological Evaluation of Pediatric Bone Tumors
The initial evaluation of any benign or malignant bone tumor usually begins with radiography. Aside from confirming the presence of a bone tumor, radiographs delineate features of the tumor to generate a differential diagnosis, guiding further imaging evaluation and management. The differential diagnosis of pediatric bone tumors can be significantly focused by the age of the patient in conjunction with the location of the lesion (Table 2.1) and radiographic characteristics that distinguish aggressive from nonaggressive lesions. Additionally, unifocal lesions versus a multifocal process can also help narrow the differential diagnosis (Table 2.2). Typically, benign lesions have a nonaggressive radiographic appearance, while malignant lesions will have a more aggressive radiographic appearance. There are certainly exceptions to this rule, including nontumoral processes such as osteomyelitis that can mimic an aggressive tumor. The radiographic appearance of an aggressive or nonaggressive tumor depends upon how the surrounding bone reacts to the underlying lesion. Nonaggressive lesions are usually slow growing and give the surrounding bone time to develop around the lesion, resulting in well-defined margins with a narrow zone of transition to surrounding intact bone. A slow-growing benign tumor can show expansion of the surrounding bone contour, without bony destruction, and without a soft tissue mass component. If a benign lesion does elicit surrounding periosteal reaction, the reaction will generally demonstrate a mature, well-formed, and less aggressive appearance.
Table 2.1
Pediatric bone tumors by their frequent locations in long bones
Epiphysis | Chondroblastoma |
Giant cell tumor (after physeal fusion) | |
Langerhans cell histiocytosis (rare) | |
Metaphysis | Aneurysmal bone cyst |
Chondromyxoid fibroma | |
Enchondroma | |
Ewing sarcoma (more commonly metadiaphyseal or diaphyseal) | |
Langerhans cell histiocytosis | |
Leukemia | |
Metastases | |
Nonossifying fibroma/fibrous cortical defect | |
Osteochondroma | |
Osteoid osteoma | |
Osteosarcoma | |
Parosteal osteosarcoma | |
Simple (unicameral) bone cyst | |
Diaphysis | Adamantinoma |
Ewing sarcoma | |
Fibrous dysplasia | |
Langerhans cell histiocytosis | |
Nonossifying fibroma/fibrous cortical defect (in older patients)a | |
Osteochondroma (in older patients)a | |
Osteofibrous dysplasia | |
Osteoid osteoma | |
Periosteal osteosarcoma | |
Simple (unicameral) bone cysta |
Table 2.2
Multifocal pediatric bone lesions
Brown tumors (hyperparathyroidism) |
Cystic angiomatosis/lymphangiomatosis |
Enchondroma (Ollier disease, Maffucci syndrome) |
Fibrous dysplasia (McCune-Albright syndrome) |
Infantile myofibromatosis |
Langerhans cell histiocytosis |
Leukemia |
Metastases |
Multifocal osteosarcoma |
Nonossifying fibromas/fibrous cortical defects |
Osteochondroma (osteochondromatosis) |
Osteomyelitis/CRMO (chronic recurrent multifocal osteomyelitis)a |
Malignant bone tumors generally demonstrate aggressive radiographic features that reflect the fast-growing nature of the lesion, limiting the ability of the surrounding bone to react or “catch up” with the underlying aggressive process. These features include a wide zone of transition with poorly defined margins as well as frank bone destruction, outpacing the ability of the surrounding bone to remodel or develop a sclerotic margin. Such an appearance can reflect soft tissue mass extension from the bone as well as a permeative or moth-eaten appearance of bone destruction. Aggressive forms of periosteal reaction also indicate a fast-growing aggressive process. These patterns include “onionskin” or multilayered periosteal reaction composed of multiple concentric parallel layers of new bone adjacent to the cortex (Fig. 2.2a), as well as spiculated periosteal reaction which can appear either as a “sunburst” pattern of divergent ossified fibers radiating out from the periosteum (Fig. 2.2b) or a “hair-on-end” pattern with ossified fibers radiating perpendicular to the cortex (Fig. 2.2c). Additionally, aggressive periosteal reaction can appear as a “Codman triangle,” which represents ossification of only the elevated periosteum at the margins of the aggressive process which then tapers down to the intact bone producing a triangular appearance (Fig. 2.2c).
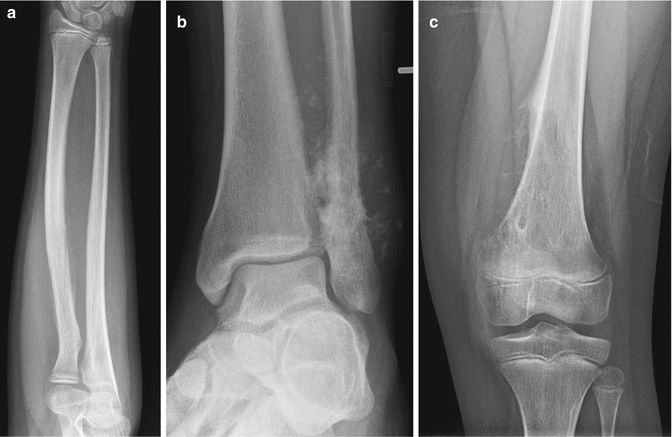

Fig. 2.2
Radiographic features of aggressive lesions. (a) This aggressive appearing lesion involving the proximal radial diaphysis demonstrates a permeative, “moth-eaten” appearance of the bone with aggressive periosteal reaction showing layers of “onionskin” appearing periosteal new bone formation as the soft tissue mass extends from the underlying bone. (b) This oblique frontal radiograph of the ankle demonstrates a lesion in the distal fibular metaphysis with a classic “sunburst” appearance of aggressive periosteal reaction. (c) An aggressive “moth-eaten” lytic-appearing lesion is seen within the metaphysis of the distal femur demonstrating bony destruction and a large soft tissue component with a “Codman triangle” at the superior margin of the soft tissue mass as well as “hair-on-end” aggressive periosteal reaction
2.2 Epiphyseal Lesions
The epiphysis generally has the least number of lesions associated with it, and this chapter will focus on chondroblastoma, Langerhans cell histiocytosis, osteomyelitis (usually subacute or chronic), and giant cell tumor (in skeletally mature patients after physeal fusion).
2.2.1 Chondroblastoma
Chondroblastomas appear as well-defined eccentric lucent lesions within the epiphyses of long bones. Additionally, they can occur in epiphyseal equivalents including apophyses and the patella (Turcotte et al. 1993). There are several radiographic characteristics which help distinguish chondroblastoma from other epiphyseal lucent lesions. On MRI, these lesions follow cartilage signal on all pulse sequences aside from occasional examples containing internal calcific matrix that demonstrates no signal. Additionally, painful lesions can demonstrate surrounding inflammatory response including bone marrow edema signal on MRI as well as bone marrow edema signal distant from the lesion (Figs. 2.3, 2.4, and 2.5). Biopsies show a benign cartilaginous neoplasm composed principally of sheets of mononuclear cells and osteoclast-type giant cells. The mononuclear cells frequently contain nuclear grooves which imparts a “coffee bean” appearance to the cells (Fig. 2.6). The presence of cartilaginous matrix and chicken-wire calcifications helps to facilitate the diagnosis. Not uncommonly, secondary aneurysmal bone cyst can develop and sometimes dominate the overall radiologic and histologic appearance to the extent that the diagnosis of chondroblastoma could be obscured (Fig. 2.6c).
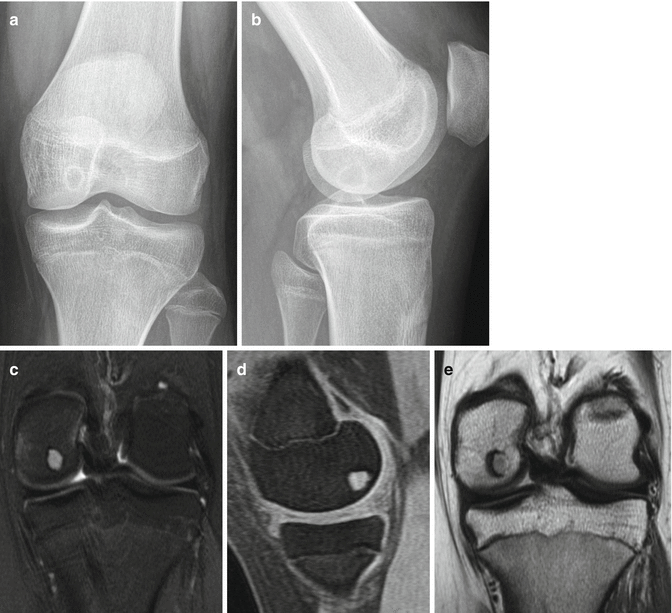
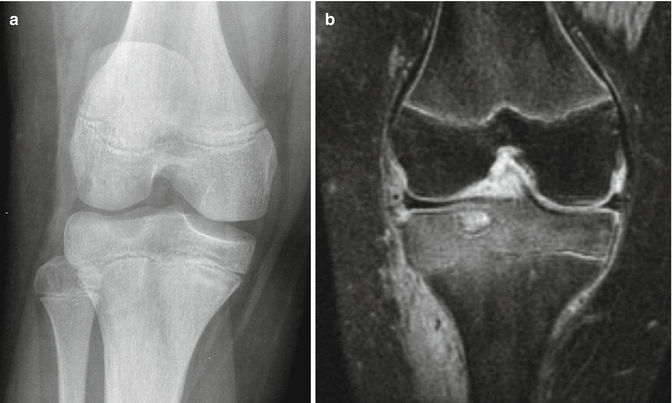
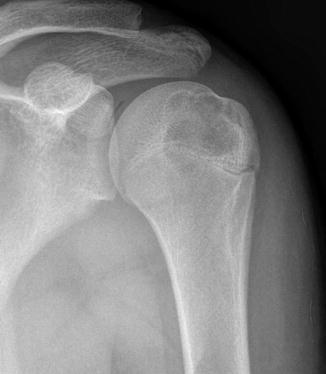
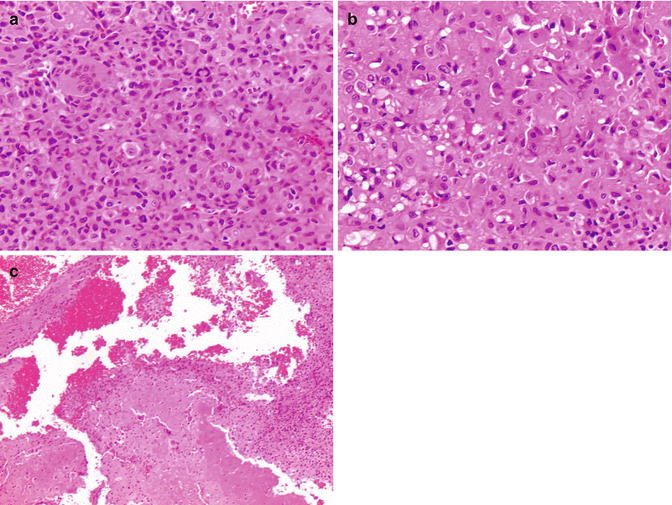

Fig. 2.3
Chondroblastoma of the distal femur. (a, b) Anterior-posterior (a) and lateral knee (b) radiographs demonstrate a lucent chondroblastoma with thin sclerotic borders within the medial condyle of the distal femoral epiphysis. (c–e) MRI sequences of the same patient (a coronal PD FS, b coronal T1, and c sagital 3C FSPGR FS) demonstrate a typical well-circumscribed lesion with surrounding low-signal consistent with the surrounding sclerosis. The lesion itself follows cartilage signal on all pulse sequences. Additionally in this painful lesion, bone marrow edema can be seen along the medial margin of the medial femoral condyle distant from the lesion itself, best appreciated on the coronal PD FS image (a)

Fig. 2.4
Chondroblastoma of the proximal tibia. (a) An anterior-posterior radiograph demonstrates a well-defined lucent lesion with sclerotic borders within the lateral proximal tibial epiphysis, abutting the cortex. (b) MRI of the same patient (coronal PD FS) shows a well-circumscribed lesion within the lateral proximal tibial epiphysis with thin surrounding low-signal consistent with the surrounding sclerosis. The lesion itself follows cartilage signal. In this painful lesion, MRI demonstrates the significant surrounding inflammatory bone marrow edema signal as well as inflammation in the surrounding soft tissues

Fig. 2.5
Chondroblastoma of the proximal humerus. An anterior-posterior radiograph of the proximal humerus shows a well-defined lucent epiphyseal lesion with well-defined mildly lobulated borders showing thin rim of sclerosis within the proximal humeral epiphysis

Fig. 2.6
Histologic features of chondroblastoma. (a) Chondroblastoma is composed of diffuse sheets of mononuclear cells admixed with osteoclast-type giant cells. (b) Prominent longitudinal grooves and “coffee bean” appearance of the cells are easily discernible, prototypic for this lesion. (c) Secondary aneurysmal bone cystlike changes in this chondroblastoma were extensive representing a histologic differential diagnosis of aneurysmal bone cyst
2.2.2 Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is included within the differential of epiphyseal lesions in the pediatric population as these lesions can occur in all locations in long bones and in many other bones including the mandible, pelvis, ribs, and spine (Fig. 2.7). Epiphyseal lesions generally appear well circumscribed and lytic in appearance, potentially resembling chondroblastoma. LCH is a spectrum of disease and can have any radiographic appearance; lesions can have both an aggressive and nonaggressive radiographic features. In biopsies, LCH is characterized by distinctive histiocytes that are reactive for CD1a, Langerin, and S-100. The characteristic cells of LCH are often accompanied by mixed inflammatory cells, typically including predominantly eosinophils (Fig. 2.8), and can show histologic similarities with osteomyelitis as discussed further in Sect. 2.3.
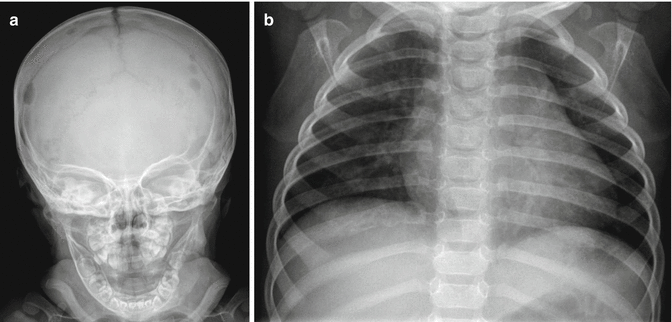
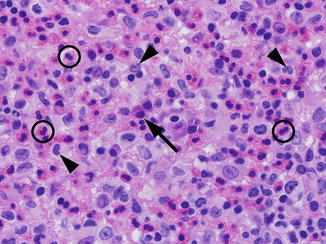

Fig. 2.7
Radiographic features of Langerhans cell histiocytosis. (a) Langerhans cell histiocytosis (LCH) involving the skull demonstrates multiple well-circumscribed lucent lesions with a “punched-out” appearance in this anterior-posterior view. Given multiple lesions, LCH is high in the differential diagnosis for this young child. (b) This anterior-posterior view of the chest demonstrates Langerhans cell histiocytosis of the rib, with an expansile lucency of the posterior right eighth rib

Fig. 2.8
Histologic features of Langerhans cell histiocytosis. The characteristic cells of Langerhans cell histiocytosis (LCH) have moderate eosinophilic to foamy cytoplasm and distinctive nuclei with folds, clefts, and grooves (arrowheads). LCH is typically accompanied by many eosinophils (circles); other inflammatory cells can be seen including plasma cells in this example (arrow). (H&E, 100× objective magnification)
2.2.3 Osteomyelitis
In tubular bones, hematogenous osteomyelitis is usually localized to the metaphysis and may spread from the metaphysis through the growth plate to the epiphysis. In children a purely epiphyseal osteomyelitis is very rare; however, in infants younger than 15 months of age, epiphyseal osteomyelitis can occur more often given that metaphyseal vessels penetrate the growth plate and enter the epiphysis, allowing entry of causative organisms. The infection is usually subacute or chronic. An epiphyseal subacute or chronic osteomyelitis will generally appear lytic with sclerotic margin, overlapping with the appearance of other epiphyseal lesions. Metaphyseal osteomyelitis is discussed further in Sect. 2.3.
2.2.4 Giant Cell Tumor
Giant cell tumor (GCT) or osteoclastoma is an uncommon neoplasm that occurs most often in skeletally mature patients with only approximately 5 % occurring before skeletal maturity, mostly in the second decade of life. While it is a benign lesion, GCT tends to show locally aggressive behavior. In skeletally mature individuals, GCT is a well-circumscribed lucent lesion that is uniformly epiphyseal in location, usually abutting the articular surface, with variable extension into the metaphysis. In the rare case of GCT in a skeletally immature patient, GCT almost always appears metaphyseal in location and usually will abut the physis.
In histologic sections, GCT is characterized by multinucleated osteoclast-type giant cells (containing up to 50 nuclei) admixed with mononuclear cells (Fig. 2.9). While other giant cell-containing lesions enter the histologic differential diagnosis, the diagnosis of GCT is usually established by appropriate clinical and radiologic context in concert with the characteristic histologic features. This correlation is particularly important in GCT. The mononuclear cell population can show variable oval, round, or spindled morphology. The proportion of the giant cells and mononuclear cells can be variable between and within lesions, and lesions with few giant cells can present diagnostic challenges, particularly in a limited biopsy specimen (Fig. 2.9d). Necrosis, mitosis, and tumor emboli in vessels can all be seen in GCT, but they do not correlate with malignant progression. Areas of reactive bone can be seen in these lesions particularly if there is an associated fracture. In a subset of cases, secondary aneurysmal bone cyst changes can occur simulating a solid variant of aneurysmal bone cyst. Immunohistochemistry is of very limited use in the histologic differential diagnosis of GCT due to the lack of a specific marker (de la Roza 2011; Dickson et al. 2008).
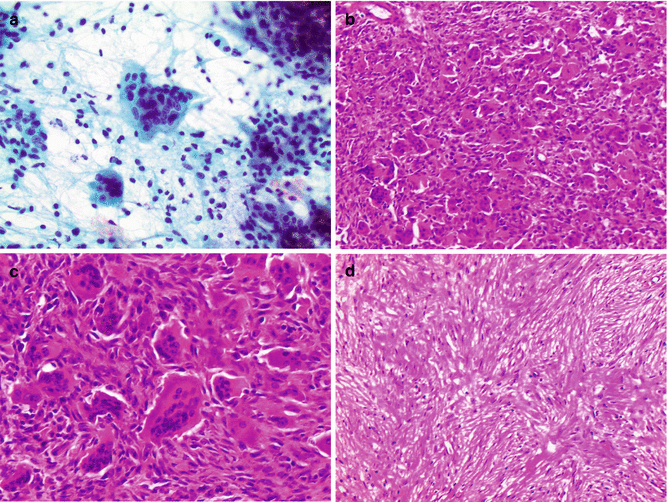

Fig. 2.9
Histologic features of giant cell tumor. (a) Papanicolaou stain of a fine-needle aspiration biopsy shows multinucleate osteoclast-type giant cells with admixed with mononuclear oval to spindle cells. (b) Histologic sections of giant cell tumor typically show numerous multinucleated osteoclast-type giant cells. (c) High-power view showing a multinucleated osteoclast-type giant cell containing 30–50 nuclei and adjacent mononuclear spindle cells. (d) A giant cell poor area within the same tumor shows a prominent spindle cell proliferation
2.3 Metaphyseal Lesions
The differing lesions that may involve the metaphysis of long bones are many (see Table 2.1). This section will discuss simple or unicameral bone cyst (UBC), aneurysmal bone cyst (ABC), osteosarcoma, Ewing sarcoma (more commonly metadiaphyseal or diaphyseal), LCH, and subacute/chronic osteomyelitis.
2.3.1 Unicameral (Simple) Bone Cyst
Unicameral or simple bone cysts (UBC) appear lucent on radiographs. They demonstrate well-defined borders with a sclerotic rim and may show mild expansion of the overlying thinned bony cortex. They usually occur centrally within the medullary space near the metaphysis and may “migrate away” from the metaphysis into the diaphysis during bone growth. Although they are called unicameral bone cysts, more mature cysts can demonstrate internal septations (Fig. 2.10). On contrast-enhanced imaging, these cysts do have a rim of vascular lining which does enhance while the internal fluid component does not. Additionally, given their internal fluid structure, a characteristic “fallen fragment sign” may be demonstrated through a pathologic fracture involving a UBC with the bone fragment layering dependently within the fluid (Fig. 2.10). Unicameral bone cysts are asymptomatic unless complicated by fracture; these lesions are the most common cause of pathologic fracture in children. UBC has a characteristic gross yellowish color and clear appearance. These lesions are often removed in piecemeal and consist of multiloculated cystic spaces devoid of true lining. The cyst wall is fibrous which may contain variable amounts of fibrin, multinucleate giant cells, and sometimes cementoid material (Fig. 2.11).
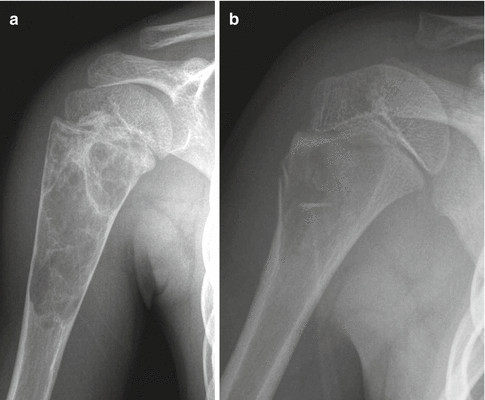
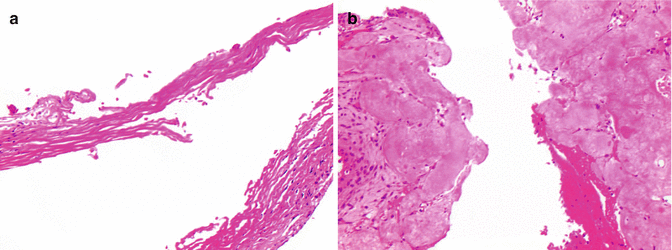

Fig. 2.10
Unicameral bone cysts of the proximal humerus. (a) Unicameral (simple) bone cyst is a benign lucent-appearing lesion often within the metaphysis with well-defined borders with no cortical destruction or periosteal reaction. This lesion demonstrates thin internal septations and mild expansion of the proximal humeral metaphysis. (b) The “fallen fragment sign” of unicameral bone cyst is the result of a pathologic fracture with cortical buckling and disruption extending through the well-defined lucent lesion. Bony fragment is seen layering dependently within the internal fluid of this unicameral bone cyst of the proximal humerus

Fig. 2.11
Histologic features of unicameral bone cyst. Unicameral bone cyst is composed of cystic spaces with fibromembranous wall (a), with deposits of fibrin-like material in the cyst wall (b)
2.3.2 Aneurysmal Bone Cyst
Aneurysmal bone cysts (ABCs) on radiographs appear lucent with sharp margins, showing a “soap-bubble” lesion with thin sclerotic borders (Fig. 2.12). The bony cortex may be very thinned to the point of nonvisualization; however, no bony destruction is seen, similar to unicameral bone cysts. ABCs are usually multiloculated and may demonstrate lacelike internal matrix. On MRI, the lesion will also demonstrate characteristic fluid-fluid levels due to layering degraded blood products, similar to UBC (Fig. 2.12c). On contrast-enhanced imaging, the internal septa and the thin inner lining of the ABC will enhance, while the multiloculated fluid-containing spaces will not (Fig. 2.12d).
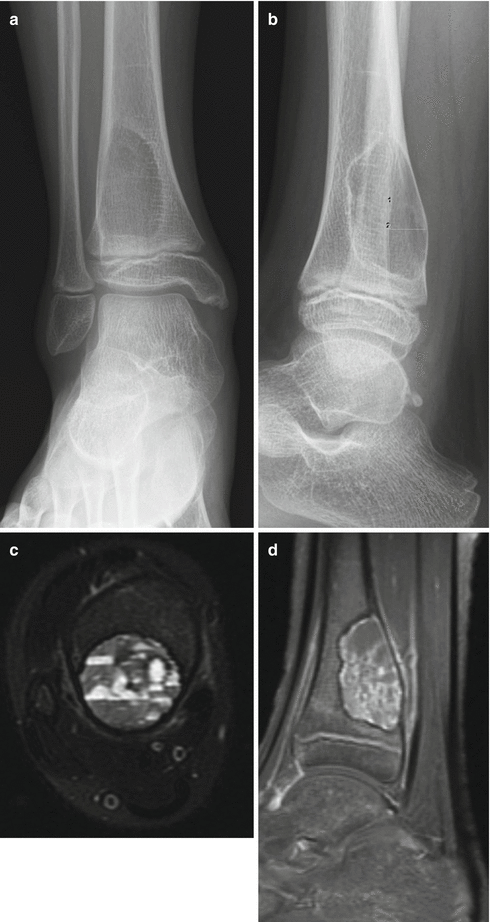

Fig. 2.12
Aneurismal bone cyst of the distal tibia. (a, b) Anterior-posterior (a) and lateral (b) radiographs of the distal tibia demonstrate an expansile lytic lesion eccentrically located and involving the posterior distal tibial metaphysis. This example has characteristic sharp geographic margins with thin rim of sclerosis and very faint lacelike internal matrix. No cortical break or destruction with no visible soft tissue component. (c, d) MRI sequences of the same patient (c axial T2WI with fat saturation, d sagittal post-gadolinium T1WI with fat saturation) demonstrate the sharp margins of the lesion, with a thin rim of decreased signal corresponding to sclerosis seen on radiographs. Multiple fluid-fluid levels within the cystic spaces, seen in panel c, represent layering of blood products and are characteristic, but not unique to aneurismal bone cysts. Typical thin enhancement of the margins and septa without a soft tissue component or cortical destruction are best seen in post-contrast T1-weighted images (panel d)
ABCs are grossly very hemorrhagic and sievelike, reminiscent of a sponge. Histologic section show multiloculated “cystic” spaces containing blood with no true lining with fibrous wall and variable amounts of osteoclastic-type giant cells, capillaries, spindle cells, and osteoid deposition that sometimes mineralize to form bone (Fig. 2.13). It is imperative to screen for secondary etiologies which may coexist in the same biopsy specimen.
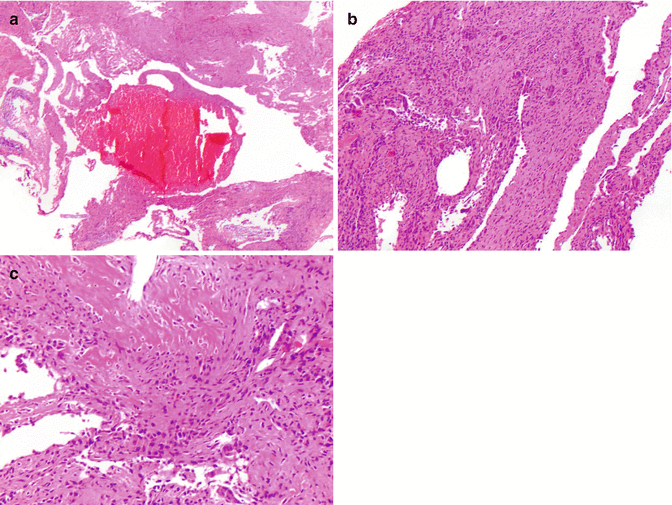

Fig. 2.13
Histologic features of aneurismal bone cyst. (a) Aneurysmal bone cyst contains blood-filled cystic spaces lined by fibrous septae. (b) Multinucleated giant cells are characteristically seen in the septae. Notably, cellular features of malignancy are absent. (c) Areas of osteoid deposition can be seen in aneurysmal bone cyst
2.3.3 Telangiectatic Osteosarcoma
An important differential diagnosis is telangiectatic osteosarcoma, which shares similar histologic features to ABCs. Telangiectatic osteosarcoma is characterized by multiple blood-filled cysts and microscopically shows tumor cells arranged along delicate networks of sinusoidal vessels. These features can mimic aneurysmal bone cyst; however, the high-grade pleomorphic cells with anaplasia, coagulative necrosis, atypical mitotic figures, and osteoid matrix are often readily identified in telangiectatic osteosarcoma (Fletcher et al. 2013). Conversely, aneurysmal bone cyst can also show osteoid formation which can undergo mineralization, representing an important histologic differential diagnosis from osteosarcoma. In these instances, the absence of malignant cytologic features is an important histologic clue to the diagnosis of aneurysmal bone cyst.
2.3.4 Conventional Osteosarcoma
Conventional osteosarcoma can often be primarily diagnosed with radiography, with other imaging modalities typically reserved for staging and planning for surgical resection. Biopsy is performed for unequivocal confirmation of the diagnosis. In radiographs, osteosarcoma typically will appear as a large lesion with aggressive features showing a mixed lytic-sclerotic appearance, ill-defined margins, as well as bone destruction and a large soft tissue mass component. As a bone-forming tumor, conventional osteosarcoma classically has dense “cloud-like” matrix easily visible on radiographs (Fig. 2.14a, b). MRI can better delineate the soft tissue component and involvement of surrounding structures, useful for surgical planning (Fig. 2.14c). Additionally, osteosarcoma classically will demonstrate aggressive patterns of periosteal reaction including “sunburst” or “hair-on-end” pattern as well as “Codman triangles.” The sunburst pattern develops when growth of the lesion outpaces the ability of the periosteum to lay down new bone, and the Sharpey’s fibers stretch out in a divergent pattern from the bone (Fig. 2.14d), whereas “hair-on-end” periosteal reaction is oriented perpendicular to the bone. It is often seen with osteosarcoma but can also develop with other aggressive bony tumors such as Ewing sarcoma or osteoblastic metastases. The Codman triangles form on the edge of the periosteum in a fast-growing aggressive lesion or process. With aggressive lesions, the periosteum does not have time to completely ossify; thus, only the edge of the raised periosteum will ossify, producing the characteristic triangular appearance along the margins of the mass. While this is found often in osteosarcoma, this type of aggressive periosteal reaction can be found in many other aggressive lesions including Ewing sarcoma, osteomyelitis, metastatic disease, and other sarcomas. Osteosarcomas can occasionally appear purely lytic and exhibit little no periosteal reaction.
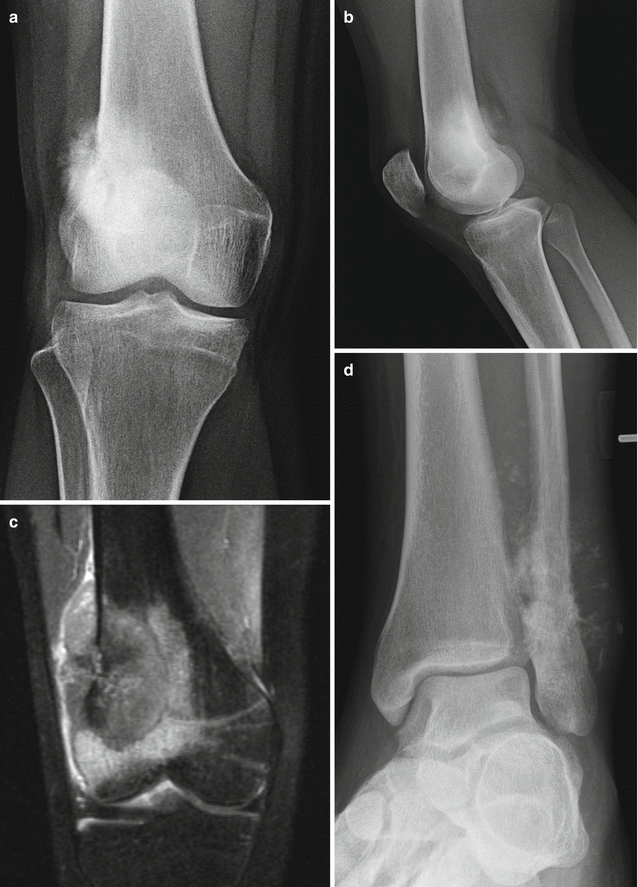

Fig. 2.14
Radiographic features of conventional osteosarcoma. Anterior-posterior (a) and lateral (b) radiographs of the knee demonstrate a large mass involving the distal femoral metaphysis with classic features of conventional osteosarcoma including dense “cloud-like” osseous matrix, cortical destruction with mass extension through the cortex. (c) Coronal T1 fat-saturated post-gadolinium imaging of the same patient better depicts the size of the soft tissue mass and extension of the osteosarcoma than radiography. Note the cortical destruction along the lateral aspect of the femur. (d) Oblique frontal radiograph of a conventional osteosarcoma involving the distal fibular metaphysis demonstrates a classic “sunburst” appearance of aggressive periosteal reaction
The histologic diagnosis of conventional osteosarcoma is established based on cells exhibiting malignant cytologic features associated with osteoid matrix material (Fletcher et al. 2013; Sanerkin 1980). Conventional osteosarcoma has a variety of histologic variants including osteoblastic, fibroblastic, chondroblastic, giant-cell rich, clear cell type, and epithelioid, yet none of these correlate with prognosis (Fig. 2.15) (Fletcher et al. 2013; Hauben et al. 2002). The malignant cells and associated osteoid permeate native bony trabeculae, often exhibiting anaplasia and atypical mitotic figures, and coagulative tumor necrosis involving the native bone trabeculae can be seen (Fig. 2.16). Osteoid matrix can be very focal or occasionally difficult to distinguish from other sclerosing sarcomas that produce thick collagen matrix. Generally, the role of immunohistochemistry in the diagnosis of osteosarcomas is limited and many pitfalls are known, including aberrant expression of markers such as cytokeratin that can mimic carcinoma (Okada et al. 2003). In cases where osteoid is scant or not easily discernible, immunohistochemical staining for SATB2 can be helpful to confirm bona fide osteoblastic differentiation (Fig. 2.17) (Conner and Hornick 2013). Recently, p16 expression has been shown to be predictive of tumor response to chemotherapy (Fig. 2.18) (Borys et al. 2012).
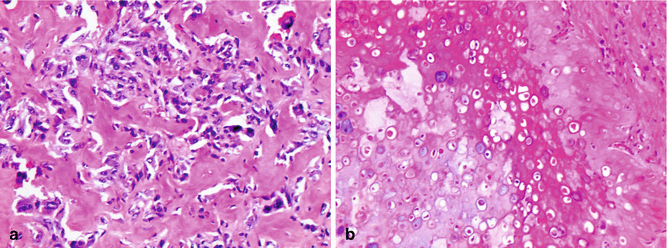
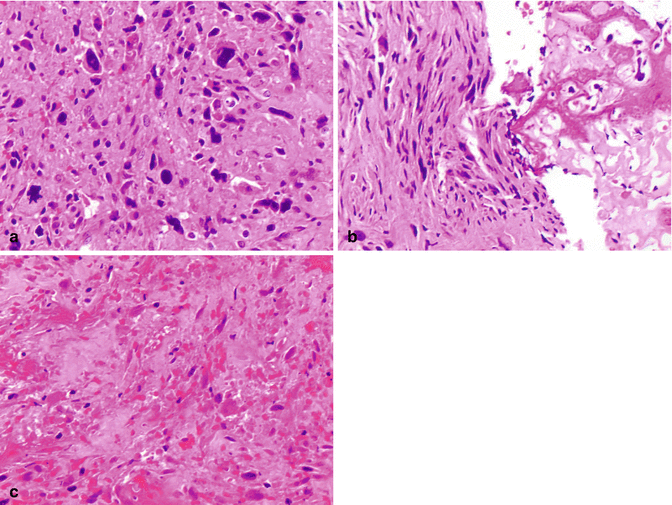
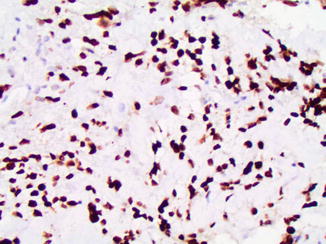
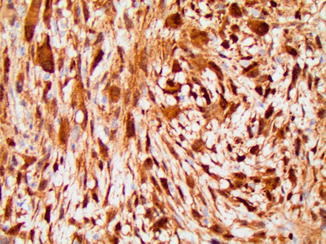

Fig. 2.15
Histologic variants of osteosarcoma. (a) Osteoblastic osteosarcoma features pleomorphic cells with abundant osteoid. (b) Chondroblastic osteosarcoma showing lobules of atypical chondrocytes merging imperceptibly with an area of osteoid deposition in the upper half of the image

Fig. 2.16
Histologic features of conventional osteosarcoma. (a, b) Conventional osteosarcoma is a high-grade malignancy characterized by cells with nuclear hyperchromasia and pleomorphism (a) and lacy osteoid material adjacent to the malignant cells (b right half of the image). (c) Coagulative tumor necrosis is a frequent feature of conventional osteosarcoma

Fig. 2.17
SATB2 reactivity in osteosarcoma. (a) SATB2 is a marker of the osteoblast lineage and is typically diffusely reactive in conventional osteosarcoma

Fig. 2.18
P16 reactivity in osteosarcoma. Diffuse nuclear and cytoplasmic P16 expression may predict a favorable response to neoadjuvant chemotherapy
Osteoblastoma, especially aggressive forms, can show histologic features mimicking conventional osteosarcoma with plump epithelioid cells that can exhibit cytologic atypia. However, unlike osteosarcoma, osteoblastoma is typically a discrete lesion often in the diaphysis and does not invade surrounding native lamellar bone.
Parosteal osteosarcoma usually involves the metaphysis of long bones, most frequently the distal posterior aspect of the surface of the tibia. By definition, in the absence of dedifferentiation, parosteal osteosarcomas are low-grade malignant neoplasms composed of spindle cells with mild atypia which may manifest a cartilaginous cap thus mimicking an osteochondroma. However, parosteal osteosarcoma lacks the orderly arrangement typical of osteochondroma (Unni et al. 1976).
2.3.5 Metaphyseal Ewing Sarcoma Versus Small Cell Osteosarcoma
Small cell osteosarcoma is a poorly differentiated variant of osteosarcoma. Similar to conventional osteosarcoma, small cell osteosarcoma most often occurs in the metaphyseal region of long bones (Ayala et al. 1989; Green and Mills 2014; Nakajima et al. 1997) and can appear more lytic, lacking the typical “cloud-like” mineralization of the conventional osteosarcoma which contains more osteoid matrix (Fig. 2.19). Ewing sarcoma is more commonly diaphyseal and will be discussed further in Sect. 2.4. However, Ewing sarcoma can also present as a metaphyseal lesion with radiographic features that mimic osteosarcoma, including similar aggressive patterns of periosteal elevation such as Codman triangle (Fig. 2.20).
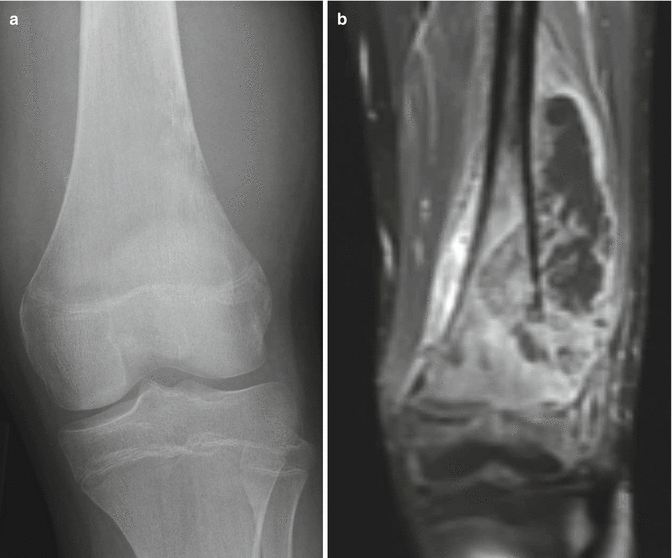
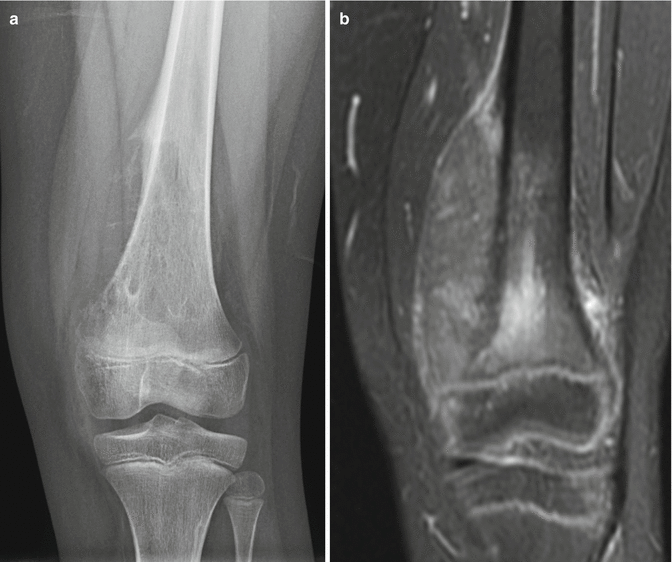

Fig. 2.19
Radiographic features of small cell osteosarcoma. (a) An anterior-posterior radiograph of the knee demonstrates an aggressive lesion with mixed lytic and sclerotic features and ill-defined margins. In this poorly differentiated osteosarcoma, the lack of osseous “cloud-like” mineralization typical of conventional osteosarcoma can overlap in appearance with an Ewing sarcoma involving the metaphysis. (b) MRI much better delineates the soft tissue component and intramedullary involvement than radiography of this poorly differentiated osteosarcoma. This coronal T1 post-contrast fat-saturated image of the distal femur shows a large aggressive destructive lesion. Note the necrotic component showing lack of central enhancement

Fig. 2.20




Ewing sarcoma mimicking osteosarcoma. (a) An anterior-posterior radiograph of this Ewing sarcoma shows features that overlap with osteosarcoma. This aggressive “moth-eaten” lytic-appearing lesion within the metaphysis of the distal femur demonstrates bony destruction and a large soft tissue component with a “Codman triangle” at the superior margin of the soft tissue mass as well as “hair-on-end” aggressive periosteal reaction. (b) A coronal post-contrast T1 fat-saturated image of the same lesion demonstrates the large soft tissue component extending from the bone, bony destruction, as well as triangular-shaped enhancement at the superior margin of the tumor at the site of “Codman’s triangle”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


