Fig. 9.1
The PCO morphology is characterized by the increased ovarian volume, by the increased number of follicles, by their peripheral arrangement, and by their small diameter
However, following these criteria, to have a polycystic ovarian morphology (PCOM), could happen to a 24% of women in the reproductive life, and this percentage could be doubled in adolescence [21], and do not permit to overcome very important diagnostic difficulties.
Our recent data on 302 healthy adolescents demonstrated that PCOM is present in 43% of group but is present in the 76% of them in the first 3 years from menarche.
After 3 years, this proportion is very different: disappears physiologically in 40% of subjects decreasing to 38%, whereas such morphology persists only in the 22% of girls after 5 years of menstrual cycle, which presumably could represent the subjects at real risk for menstrual dysfunction or PCOS (Fulghesu AM submitted for publication).
The spontaneous evolution versus the normal ovarian morphology suggests that PCOM in this age represents a developmental step in ovarian function.
Other US aspects, as increased ovarian stroma and higher stromal blood flow, whereas accepted as significant predictors of hyperandrogenism [22, 23], are not suggested in the official guidelines for the diagnosis of PCOM, but could be helpful in identifying the syndrome.
Indeed, excluding the evaluation of the ovarian stroma, it excludes the parameters that were already considered the most specific to the strong correlation with the circulating androgens [24]. In particular, in 1985 Adams and coworkers had reported the peripheral disposition of the follicles in the ovary PCO around a hyperechoic stromal tissue core. Dewailly observed that the choice of studying the ovarian volume is due to the fact that this parameter not only is easy to measure, but is also directly correlated with the hypertrophy of the stroma, of which discouraged the direct evaluation because it was considered subjective and difficult [25, 26]. For these reasons in recent years numerous studies have been undertaken to improve the diagnostic US specificity mainly focusing on evaluating stromal hyperplasia. Various systems have been proposed to define the increase of representation and echogenicity of stroma, normally slightly lower than that of the myometrium. None of these proposals has had large following because it is considered a highly subjective evaluation and operator-dependent instrumentation.
In 2001, my group has evaluated the measure of stroma compared to the remaining ovarian parenchyma, measuring the picture corresponding to the maximum ovary planar section, the area of the central stromal thickening zone (drawing obtained the peripheral profile of the stroma with caliper) and the total area of the ovarian parenchyma (drawing obtained with a second caliper, the outer limit of organ) and then calculating the ratio (S/A) (Fig. 9.2) [27].
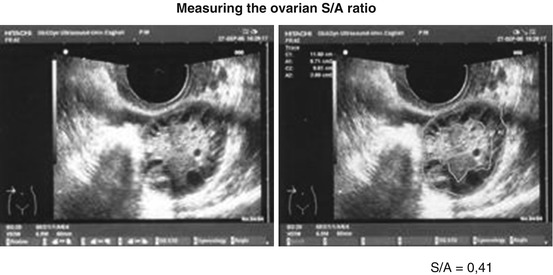

Fig. 9.2
A proportion between the ovarian area in median section (A) and stroma area in the same section (S), S/A ratio is obtained from two measures given by caliper
With this kind of evaluation the diagnosis of ovary PCO corresponds to values of S/A > 0.34, more than a third of the ovary area in the median section (Fig. 9.2).
A subsequent multicenter study [24] indicated the S/A ratio, is gettable by standard technology, without inter-operator variations and provides great sensitivity and diagnostic specificity (96%). This index closely correlated with the plasma testosterone (R 0.731 p < 0.001) or/and androstenedione (R 0.734 p < 0.001).
The adoption of this new parameter finally could lead to a precise differentiation of PCO ovary already named multifollicular ovary. The multifollicular pattern (MFO) is described by several authors as an evolutionary step in adolescence [28] or as pathognomonic of amenorrhea or oligomenorrhea [29]. Such situations, from the pathogenic point of view, are characterized by gonadotropin pulsatility alterations due to eating disorders, or strong physical or psychological stress [10, 30] (Fig. 9.3).


Fig. 9.3
A proportion between the ovarian area in median section (A) and stroma area in the same picture (S), S/A ratio is obtained from two measures given by caliper. Ovary PCOM in not PCOS girl: S/A 0.11
These secondary amenorrhea are frequent in young age (adolescents often stressed and with mild eating disorders (DCA)), but with no signs of hyperandrogenism. This disorder is increasing and can be associated with normal BMI in the presence of conflicting attitudes towards food.
Other authors [16, 31, 32] confirmed the importance of S/A stroma in diagnosing PCOS. Battaglia and Sun found out exactly the same S/A ratio cutoff respectively in 3D and transrectal US studies, and, recently, the S/A ratio demonstrated the best US PCOS diagnostic performance when associated with Total Ovarian Follicular count (FNPO) [33].
The increased stroma, until now, is studied mostly in adult population, for the difficult evaluation in TA. Further studies could confirm this possibility, considering that technical probe and software amelioration could overtake the problem of the TA US approach.
9.3 Additional Diagnostic Perspective
In recent years, to try to improve the ultrasound diagnosis of PCO, the application of color Doppler in the transvaginal US was studied in ovarian and uterine vessels highlighting an increase of pulsatily index of the uterine artery for effect of high levels of androgens and a reduction of uterine perfusion [33].
Subsequently the focus shifted on the vessels of the ovarian stroma noting the association between high levels of LH and increased stromal vascularization with a decrease of the intraovarian resistances and consequent stromal hyperplasia in patients with PCOS pattern [22].
Higher stromal blood flow, whereas accepted the significance as predictors of hyperandrogenism, actually is not suggested in the diagnosis of PCOS [7].
On the other hand, PCOS subjects presented increased Anti-Mullerian Hormone (AMH) levels [34, 35]. The number of follicles at all growing stages especially pre-antral and small antral follicles is increased in PCO. Thus elevated serum AMH level, as a reflection of this follicular stock, is two to fourfold higher in women with PCOS than in healthy women [35, 36]. Given its strong implication in the pathophysiology of PCOS, serum AMH had been considered the “Gold Standard” in the diagnosis of PCOS. Even though serum AMH would be theoretically more accurate than antral follicular count (AFC), as it reflects also the excess of small follicles non-visible on ultrasound [37], it is considered premature to make this diagnostic transition.
The robust association between AMH and AFC has led some authors to insert their performance in the diagnosis of PCOS [38], but it is found in all PCOM populations also in absence of hyperandrogenism [39].
In conclusion, therefore, we can say that the diagnostic ultrasound of PCOS cannot ignore the rules established in Rotterdam in 2003, which at present constitute the major criteria for identification of PCOS ovary; however, these guidelines are difficult to apply during the first 5 years after menarche when the physiological PCOM presence can reach 2/3 of the subjects.
9.4 Clinical Criteria
When PCOS is suspected in adolescence, a collection thoughtful and careful of medical history will be very useful for verifying the durability and authenticity of the described symptoms, and identify the presence of risk factors for PCOS and insulin resistance as by low birth weight “sine causa,” big weight gain in the first year of life, and finally pubarche premature and/or precocious puberty [40, 41].
Irregular menstrual cycles with oligoanovulation or secondary amenorrhea, is a very frequent symptom.
This event is to be considered normal in the first years after menarche, and is shrinking gradually from 2 to 3 years of gynecological life in normal girls, while it can stabilize with the passing years in individuals suffering from PCOS [42].
This clinical sign is present also in a great number of subjects stressed, athletes, too lean, or suffering from some form of eating disorders, as orthorexia, which presented menstrual dysfunction for alteration of gonadotropin secretion. For this reason, it is important to evaluate the lifestyle of subjects [43].
In adolescence, even the classic clinical criteria of hyperandrogenism such as acne, hirsutism, and alopecia should be considered with a different approach.
In fact, acne is a teenage phenomenon, physiological in both sexes. Its onset follows the adrenarche and adrenal androgen production, and physiologically tends to shrink after 2–3 years after menarche, to disappear in 6–7 years. In subjects really PCOS on the contrary, it is getting worse and as severity of injuries as an extension, but especially no signs of spontaneous improvement, and returns to the suspension of any treatment even up to 35–40 years [44].
On the contrary rarely hirsutism is a teenage temporary phenomenon. In fact, it needs a long time of hyperandrogenism to become a real problem. It may have different etiologies in addition to PCOS, first of all adrenal hyperfunction by enzyme deficiencies, but also a fair incidence of family forms.
For a correct assessment of hirsutism the Ferriman and Gallway [45] is the best scale, identifying nine body areas where hair follicles are hormone-dependent, with the exception of leg and forearm, where the familiar ethnic component is predominant. This scale assigns a value from 0 to 4 for each area and considers three levels of severity depending on the score achieved: <8 not relevant; Mild from 8 to 14; moderate 15 to 24; serious >24 (Fig. 9.4).


Fig. 9.4
Assignment of hirsutism score
Hyperandrogenic alopecia is really rare in young girls, and the differential diagnosis with other familial forms or “sine causa” can be difficult. For this reason its use in the diagnosis of PCOS in adolescence is marginal.
9.5 Endocrine Criteria
The endocrine assessment must include androgens assay. Androstenedione and Testosterone, adrenal hormone 17OHP, and prolactin. Gonadotropins may be evaluated in suspicion of hypothalamic–pituitary axis disorders for example DCA or stress, or other primary ovarian disease.
Classically, the syndrome was attributed to altered LH/FSH secretion. Recent data have shown that the relative increase in the LH/FSH is only present in a minority of cases of PCOS [46], and does not alter either the prognosis or the therapeutic approach [47, 48].
The estradiol assay can be useful only in that it indicates the presence of follicular activation.
Prolactin is essential in the differential diagnosis of anovulation due to hyperprolactinaemia.
Ovarian androgens can be assessed only in early follicular phase, being involved in the production of progesterone, and therefore always high during ovulatory and luteal phase.
Often total testosterone (T) and androstenedione (A) are not so high in absolute values, or only one rose above normal cutoff (A > 3.5 ng/mL, T > 0.7 ng/ml), as a function of individual enzymatic pathways. Moreover, the dosage of the T is not technically simple and can be unreliable. It would be useful to combine it with the dosage of SHBG, which permits to calculate the Free Androgen Index (FAI) assessment, which replaces the direct determination of free testosterone (FT). FAI is direct active on receptors and is considered the most reliable marker of hyperandrogenism [47].
Among the adrenal androgens the most important is the 17OHP, which allows the exclusion of cases of classical and non-classical Congenital Adrenal Hyperplasia (CAH) and, in case of clinical adrenal hyperfunction, DHEAS.
9.6 Metabolic Aspect
The incidence of obesity, metabolic disorders, diabetes type 2, and the presence of metabolic syndrome was significantly increased in patients with PCOS [46].
Often the adipose tissue presents an android distribution, similar to an apple, which is put on relation with both insulin resistance and hyperandrogenism [49] and accounts for metabolic and cardiovascular disease. Obesity was observed in about half of women PCOS during childbearing age [50] and is considered one of the causes of insulin resistance and hyperinsulinemia.
Hyperinsulinemia, in response to food ingestion, however, affects also a 40–60% of normal-weight subjects presenting normal fasting insulin levels [51]. It’s been suggested that normal-weight women with PCOS are suffering from a form of insulin resistance “intrinsic” to the syndrome while the obese patients present a state of insulin resistance in part inherent to the syndrome, and, in part, determined by increased body fat. Increased insulin secretion and peripheral insulin resistance may coexist in a heterogeneous way depending on the BMI.
Hyper-insulin secretion may be different, from a pathophysiological point of view, in lean and obese patients. From clinical observations in subjects presenting low birth weight and premature adrenarche and showing insulin resistance in childhood and young age, some authors consider this state a risk factor for development of PCOS at puberty [40, 41, 52].
In adolescence, fat deposits must reach 24% of the body mass to have menarche [43], and from a metabolic point of view, it is linked to a functional development of insulin resistance, which should be temporary and run out about 2 years after menarche. Often subjects with PCOS do not lose this metabolic characteristic and present multiple endocrine, skin, and biochemical effect of hyperinsulinemia [44]. However, despite this metabolic factor it is universally recognized as an important element of pathophysiology of the syndrome, to date it is not considered on Guidelines on Metabolic diagnosis.
This diagnosis should be made with both the determination of insulin and fasting glucose and HOMA calculation, which discloses peripheral insulin resistance and is increased especially in presence of body fat excess, and glucose and insulin under Oral Glucose Tolerance Test (OGTT) for evaluating the insulin response after load [53], which may be present also in lean subjects. In adolescence we find blood glucose curves almost always normal, in view of the large secretory capacity of the pancreas, except in cases of severe obesity, whereas the increased insulinemic response to glucose load is present in 70–90% of obese and 50% of lean subjects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


