Figure 2.1 Biliary duct development timeline.
Other components of the liver parenchyma have different origins. Thus, Kupffer* cells appear at about 5 weeks’ gestation, apparently from outside of the liver. Mesodermal cells from the early liver anlagen form the mesenchymal framework of the liver, including its perisinusoidal Ito† cell population.
Following these early phases of liver development (induction, migration and formation of hepatocyte cords and hepatic ducts), there is another distinct phase of early postnatal liver development characterised by cell maturation, hepatocyte proliferation and expansion of the liver volume, possibly controlled by activation of the canonical Wnt signalling pathway [13]. During this phase, the intrahepatic bile ducts also elongate from the centre to the periphery.
2.1.1 Haematopoiesis
The liver does not generate haematopoietic cells de novo but is colonised by haematopoietic stem cells, probably from the yolk sac initially, which expand and mature within the developing liver and make anything up to two-thirds of the liver volume within the second trimester. Primitive erythroid cells migrate to the fetal liver and have a dramatic upregulation of adhesion molecules that allows them to bind to fetal liver macrophages. The first haematopoietic stem cells to enter the liver are pluripotent and can form any haematopoietic cell. Their first step in intrahepatic maturation is to commit to a more limited range of lineage options, typically as either an erythromyeloid precursor or a common myelolymphoid progenitor. Nevertheless, erythropoiesis predominates at this stage. This process declines and stops towards the end of gestation with the bone marrow and spleen taking over. At this point, hepatocytes upregulate the expression of metabolic and detoxifying enzymes.
2.1.2 Vascular events
Vascular structures in the developing liver are
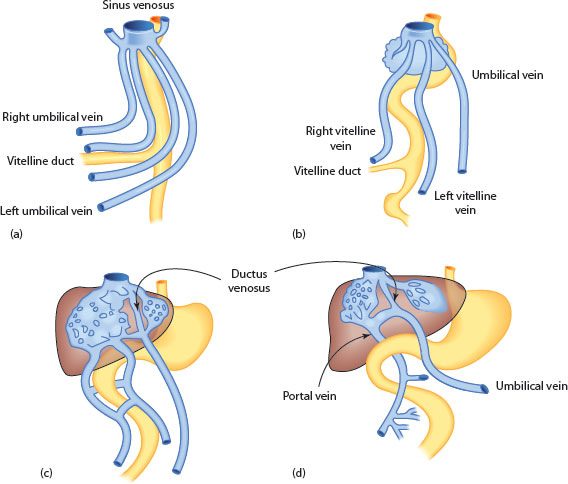
1. Vitelline‡ veins (paired): These carry blood from the gut to an evolving sinusoidal plexus and are originally from the yolk sac. These veins initially have a number of extrahepatic interconnections, resembling a stepladder, which then undergoes remodelling to form a single but now S-shaped portal vein, lying behind the distal foregut (Figure 2.2).
Figure 2.2 (a) Development of portal vein. Initial stage of two paired veins (umbilical and vitelline) draining into the sinus venosus. (b) Atrophy of right umbilical vein and vitelline duct. (c) Emergence of sinusoids in liver fed by interconnecting vitelline veins and umbilical vein. (d) Emergence of single portal vein. Umbilical vein connected to suprahepatic veins by ductus venosus.
BOX 2.2: Ductus venosus of Arantius*
This structure is present in many mammalian species, although it has disappeared by term in horses and pigs. In humans, it is a branch of the left portal vein opposite the origin of the umbilical vein and empties into the left hepatic vein (usually). About 20%–30% of umbilical blood flow is shunted through the ductus venosus in the fetus, with progressive diminution as gestational age progresses. The presence of an actual sphincter at its junction with the umbilical vein is controversial [14], although it is capable of dilatation or constriction in response to catecholamines, for instance [15]. The usual reason for this is fetal hypoxia, and diversion of blood away from the liver may be one cause of intrauterine growth retardation. Complete functional closure can be seen in >90% of infants by 2 weeks’ postnatal age as assessed by Doppler scans [20].
Persistence of the ductus venosus has a male predominance, and the resulting portosystemic shunt can be assessed by plasma ammonia and galactose levels and may cause neurocognitive impairment. (See Chapter 22.)
* Giulio Cesare Aranzi (1529–1589), Italian anatomist.
2. Umbilical veins (paired): Carry oxygenated blood from the placenta to the right side of the developing heart at the sinus venosus. The right umbilical vein disappears early in gestation, leaving the other enveloped by the liver. This joins the left portal vein, allowing oxygenated blood into the sinusoids, although the majority of blood flow is directed through a distinct low-pressure venous channel, or ductus venosus, between the left portal vein and the hepatic vein confluence (Box 2.2). This structure is rich in innervated smooth muscle cells and has a condensation of elastin fibres at its origin from the portal sinus, appearing as an ‘hourglass’ in anatomical study and on ultrasound [14]. Although there is no actual sphincter, it does have functionality [15]. There is closure at the time of birth, although it can still be negotiated with catheterisation for a variable time postnatally before subsequent fibrosis and later atrophy. Externally, its course can be appreciated as the site of attachment of the lesser omentum on the undersurface of the anatomical left lobe.
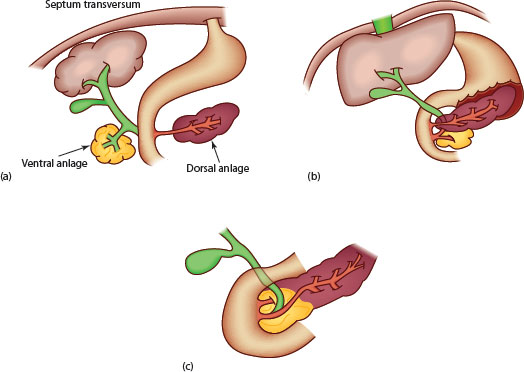
Figure 2.3 Development of pancreas. (a) Initial separation with ventral anlage attached to the developing biliary duct. (b) Rotation of ventral anlage and bile duct behind the duodenum. (c) Fusion of the pancreatic anlagen with crossover of the dorsal duct to now drain through the ventral orifice.
3. Posterior cardinal veins (paired): These carry blood back from the lower half of the body along the posterior body wall. These become the paired azygous* and hemiazygous venous system in the adult.
The larger inferior vena cava is embryologically a much later structure and is formed from many different venous precursors, for example, the subcardinal
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree