42 Development of the Cardiovascular System
Embryologic development of the heart requires the coordination of multiple steps, including heart tube formation, cardiac looping, chamber septation, and development of appropriate inflow and outflow tracts. Additionally, the cardiac conduction system and coronary artery system must develop during this time period. After organogenesis is complete, normal fetal circulation is notable for the presence of three levels of communication that normally close after birth, namely the ductus venosus, the foramen ovale, and the ductus arteriosus. Although these communications are vital for fetal circulation, they can be maladaptive in the postnatal period. Functional closure of all three communications generally occurs during the transition to postnatal life. Understanding the complexity of the normal development of the cardiovascular system allows for greater understanding of the development of congenital cardiovascular disease (see Chapters 43 and 44).
Embryologic Development
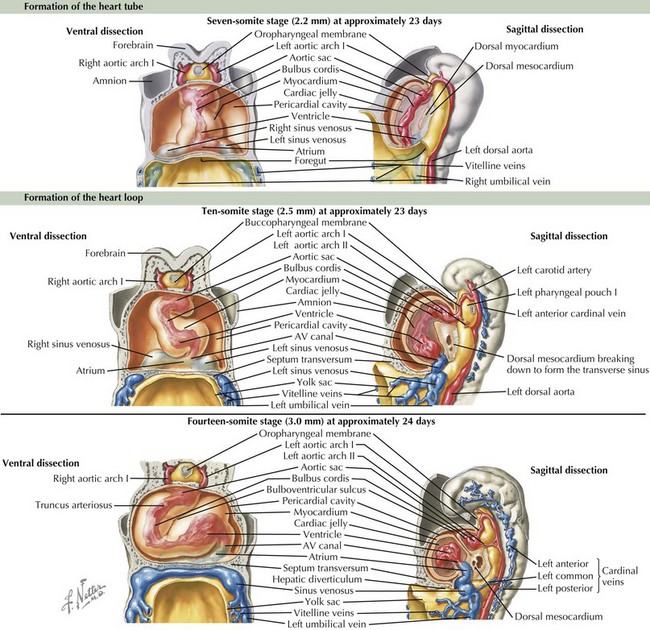
Heart Tube Formation
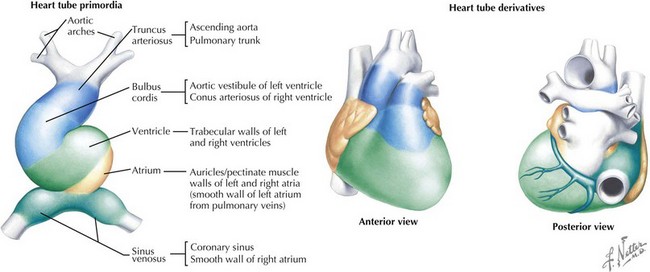
The cardiovascular system is derived primarily from the splanchnic mesoderm, which develops on the 15th day after ovulation. Migrating cells form the cardiogenic crescent, which fuses in the midline as embryonic folding occurs, creating the heart tube by day 20 to 21 of development. The heart begins to beat on day 22 of development. At this time, the heart tube segments begin to differentiate, with constrictions demarcating specific segments of the tube (Figure 42-1). Most cranially, the bulbus cordis leads into the truncus arteriosus, which connects to the aortic sac, the aortic arches, and the dorsal aorta. Caudal to the bulbus cordis is the primitive ventricle followed by the primitive atrium, which is connected in turn to the sinus venosus. Between these segments, transitional zones exist that will become septa, valves, conduction tissue, and fibrous skeleton structures.
Cardiac Looping
The crucial process of cardiac looping evolves over day 23 to 28 of gestation, with the bulbus cordis of the heart tube bending ventrally, caudally, and toward the right of the embryo, forming a D-looped heart (see Figure 42-1). This positions the proximal bulbus cordis to become the future right-sided right ventricle and the primitive ventricle to become the future left-sided left ventricle while also positioning the atria dorsal to the future ventricles (Figure 42-2). The process and direction of looping appear to be under genetic control within the myocardium, with dysregulation of this process potentially resulting in abnormal positioning of the four chambers of the heart, such as occurs in an L-looped heart (i.e., ventricular inversion). Through looping, the transitional zones are brought together along the inner curvature, and the primitive chambers are more predominantly located along the outer curvatures. Shortly after the completion of looping, blood begins circulating within the embryo and cardiac septation begins, as does the formation of the aortic arches.
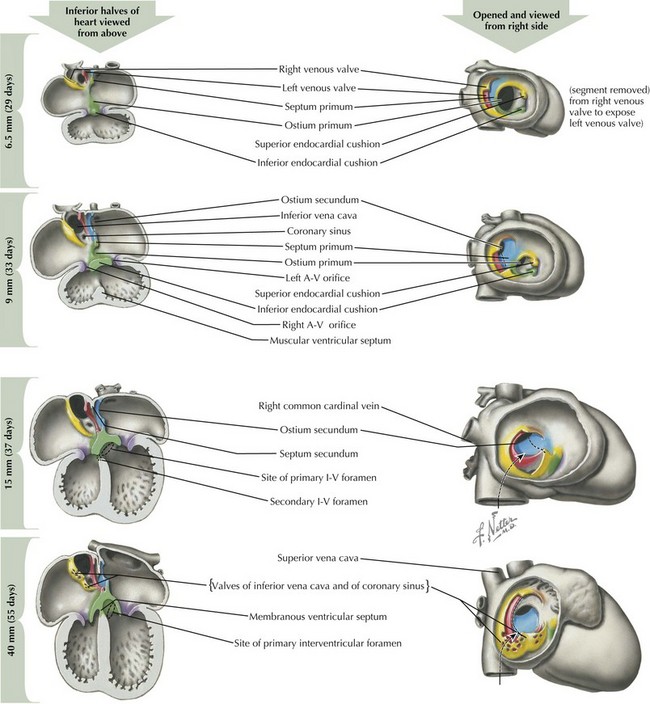
Endocardial Cushion Development and Atrial Septation
After looping has completed, the division of the four chambers and ventricular outflow tract must begin through division of the atrial and ventricular structures via the endocardial cushions, and division of the right and left sided structures through a complex process of septation (Figure 42-3). The endocardial cushions develop early in this process, appearing at the level of the future atrioventricular (AV) valves. Inferior and superior endocardial cushions grow toward each other, dividing the common AV canal into left and right AV openings. In so doing, they establish the site of the future AV valves, which will develop between the fifth and eighth weeks of development through a process of invagination of the cardiac wall.
Around day 26 to 28, atrial septation begins with formation of a crescent-shaped membrane from the posterior-superior wall of the atria, called the septum primum. On day 35, septum primum begins to extend toward the fusing endocardial cushions, gradually separating the left atrium from the right atrium. The endocardial cushions of the AV canal continue to develop and separate the atria, closing the ostium primum (see Figure 42-3). Failure of the aforementioned processes results in AV canal defects, frequently associated with trisomy 21. Classical embryologic teaching has been that before completing the division between the two atria, perforations develop in the septum primum, creating the foramen secundum, allowing continued flow between the two atria. However, virtually all hearts that have a septum primum display a crescentic superior edge with attachments on both ends of the crescent and no remnant of septum primum above the crescent indicating that there is no hole within septum primum but rather that the crescent shape was there originally.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree