44 Cyanotic Congenital Heart Disease
Clinical Presentation And Evaluation
Transposition of the Great Arteries
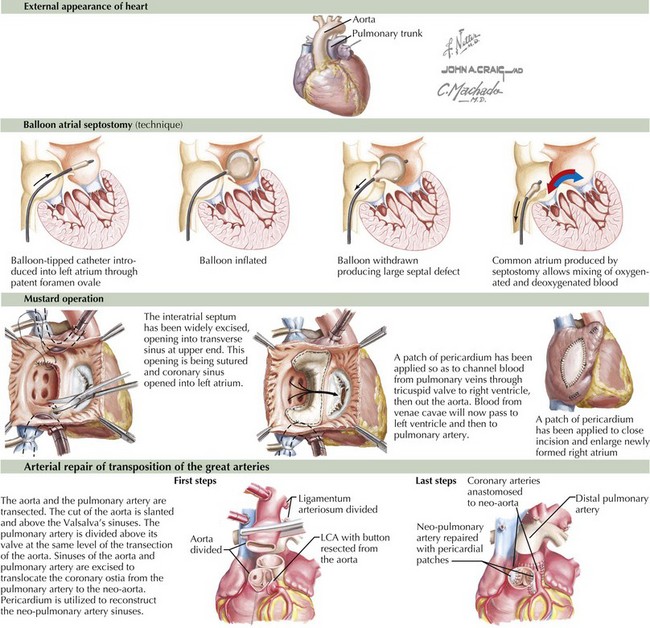
Transposition of the great arteries means that the pulmonary artery arises above the left ventricle and the aorta above the right ventricle (Figure 44-1). It is the most common cardiac cause of cyanosis in the neonatal period (0.2-0.4 in 1000 live births), accounting for 7% of congenital heart defects. Its incidence is increased in infants of diabetic mothers. It is not seen in patients with DiGeorge syndrome. Cyanosis results because the pulmonary and systemic circulations flow parallel to one another with minimal mixing: deoxygenated systemic venous blood returns to the right atrium and right ventricle and out the aorta, and oxygenated pulmonary venous blood returns to the left atrium and left ventricle and exits into the pulmonary arteries. These two parallel circuits are compatible with survival only because there is some point of mixing, usually at the foramen ovale.
Immediate intervention is usually necessary to augment the interatrial shunt. This is accomplished through a Rashkind balloon atrial septostomy (see Figure 44-1). This procedure allows for increased mixing, resulting in tolerable aortic saturations. Surgery remains the definitive therapy. Historically, this was accomplished through an atrial switch operation—Mustard (see Figure 44-1) or Senning procedures—redirecting inflow from the pulmonary veins to the right ventricle and from the venae cavae to the left ventricle. Perioperative mortality was low, but concerns regarding arrhythmia and the durability of the right ventricle (which remains the systemic ventricle) led to a different surgical approach, with a higher degree of technical difficulty but improved physiology. Currently, the preferred method is the arterial switch operation in which (1) the aorta is bisected and the distal aorta is brought beneath the bifurcation of the pulmonary trunk while the pulmonary trunk is displaced anteriorly (the Lecompte maneuver) where the aorta is anastomosed to the former pulmonary trunk and the main pulmonary artery is anastomosed to the former aortic trunk, (2) the coronary arteries are detached from the former aortic trunk and reimplanted on the pulmonary trunk (the new aortic root) with pericardial patches placed on the sites where the coronary arteries were harvested, and (3) the ASD is repaired (see Figure 44-1).
Tetralogy of Fallot
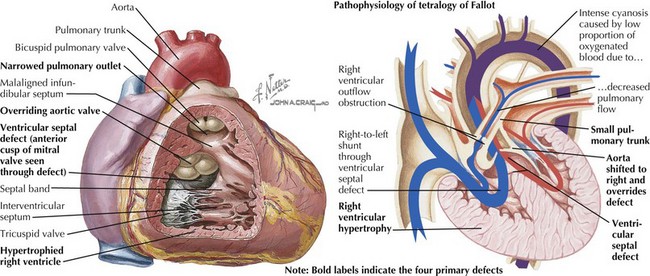
Tetralogy of Fallot is characterized by an abnormally small subpulmonary conus or outflow tract, resulting in anterior and cephalad displacement of the infundibular (outflow tract) septum. This produces the four characteristic findings: (1) subvalvular pulmonic stenosis, (2) VSD caused by malalignment of the infundibular septum relative to the rest of the ventricular septum, (3) “overriding aorta” (i.e., the aortic valve sits above both ventricles), and (4) right ventricular hypertrophy caused by pressure overload from the large VSD (Figure 44-2). It occurs in 0.19 to 0.26 in 1000 live births and represents 8% of congenital cardiac lesions. Tetralogy of Fallot is found in more than 25% of patients with DiGeorge syndrome and chromosome 22q11.2 microdeletion. The incidence of tetralogy of Fallot is higher than the general population in children of mothers with phenylketonuria and occurs more frequently in children with thrombocytopenia absent radii syndrome. Associated cardiac defects include right aortic arch (25% of patients) and ASD (10% of patients).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree