A patient with Cushing’s syndrome before (a) and after (b) adrenalectomy. Note the typical round “moon” face in (a). Picture courtesy of [24].
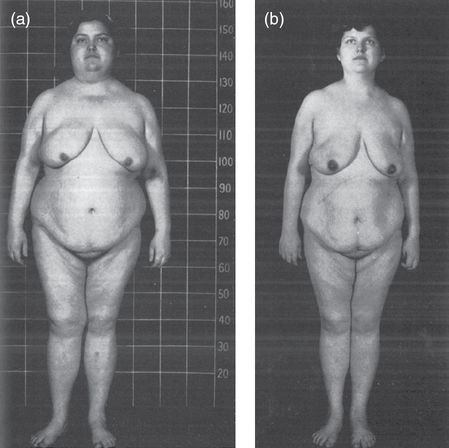
Patient with Cushing’s syndrome before (a) and after (b) adrenalectomy. This patient depicts the Cushingoid habitus in (a) with central obesity, peripheral wasting, and a round “moon” face. Picture courtesy of [24].
More discriminatory | Less discriminatory | ||
|---|---|---|---|
Feature | Frequency (%) | Feature | Frequency (%) |
Facial plethora | 90 | Obesity/weight gain | 95 |
Thin skin/striaea | 70–90 | Rounded face | 90 |
Easy bruising | 65 | Reduced libido | 90 |
Proximal myopathyb | 60 | Menstrual irregularity | 80 |
Osteoporosis | 50 | Hypertension | 75 |
Hirsutism | 75 | ||
Psychiatric symptoms | 70 | ||
Impaired glucose tolerance | 60 | ||
Cortisol exerts an anabolic effect on the liver, stimulating gluconeogenesis and glycogenesis, and a catabolic effect on muscle and adipose tissue, stimulating proteolysis and lipolysis. The result is the classic Cushingoid habitus: central fat deposition with peripheral wasting.
There is progressive fat deposition in the abdomen, face (including retro-orbital), and neck. Fat accumulation in the face gives rise to a round face (the “moon face”), while fat accumulation in the neck, specifically the supraclavicular and dorsocervical fat pads, is termed a “buffalo hump.” Central obesity is the most sensitive manifestation, and often the first. Obesity is only one component of a metabolic syndrome seen in many patients characterized additionally by hypertension, diabetes mellitus type 2, and hyperlipidemia. Acanthosis nigricans is a possible manifestation of insulin resistance.
The signs and symptoms of protein catabolism are of high discriminatory value to the clinician, as they are most specific [26–28]. Patients may complain of muscle weakness, specifically proximally, which may manifest as the inability to climb stairs or rise from a seated position. Decreased bone formation, increased bone resorption, and decreased intestinal and renal calcium reabsorption may lead to osteoporosis manifesting as fractures. A cortisol-mediated inhibition of collagen synthesis results in skin atrophy that appears translucent and is susceptible to tearing. Poor dermal support and reduced elasticity of the vessel walls make patients especially susceptible to bruising in response to minor trauma, which manifests as ecchymosis. Dermal tears lead to atrophic scarring and violaceous striae, which are pathognomonically more than 1 cm wide and located on the abdomen and flank. Facial plethora is a consequence of a thinned epidermis.
Hypogonadism results from cortisol’s inhibitory effect on the release of gonadotropin-releasing hormone from the hypothalamus. It may manifest clinically as menstrual irregularities (menorrhagia, amenorrhea), infertility, and reduced libido. A hyperandrogenic state, common to ACTH-dependent Cushing’s syndromes, may present as hirsutism, acne, and temporal hair loss.
A wide range of emotional disturbances is associated with cortisol excess, including, but not limited to, depression, hypomania, frequent mood swings, psychosis, insomnia, and short-term memory loss. Depression is most common, and residual psychiatric symptoms may persist even with normalization of cortisol [29].
Glucocorticoids inhibit the synthesis of arachidonic acid, a key molecule in the inflammatory cascade, and suppress cell-mediated immunity. Cushing patients are at increased risk for infections, particularly invasive and cutaneous mycoses.
Patients with aggressive ACTH-secreting tumors may present without the classic Cushingoid appearance. High levels of cortisol overwhelm 11β-hydroxysteroid dehydrogenase type 2 in the renal tubules and exert their effect on the mineralocorticoid receptors. These patients present with the acute onset of severe hypertension, hypokalemia, hyperglycemia, and myopathy. There may be no weight gain.
Iatrogenic Cushing’s syndrome will present similarly to endogenous Cushing’s syndrome, but without hyperandrogenism and with an increased incidence of glaucoma, cataracts, and avascular necrosis [3].
Morbidity and mortality
Women with Cushing’s syndrome have a more than twofold increased mortality risk [30,31]. While this risk has been well established for patients with Cushing’s disease, it is currently unclear whether the same applies for those with an adrenal Cushing’s syndrome as well [32,33]. There are currently no good data on patients with malignant Cushing’s syndrome, such as adrenal carcinomas and ectopic ACTH syndrome, but these patients generally have poor prognoses [10].
The cardiovascular complications of Cushing’s syndrome – coronary artery disease, congestive heart failure, and myocardial infarction – contribute greatly to morbidity and mortality. These are likely the result of a hypercortisol-related metabolic syndrome. Further, there is often some persistence of the metabolic syndrome and cardiovascular risk factors in patients with long-term normalization of cortisol [34,35]. It is inconclusive at this time whether patients in remission from Cushing’s syndrome still have increased mortality. However, persistence or recurrence of disease is a main determinant of mortality in this population [31,33]. Collectively, these conclusions form the rationale for early diagnosis, prompt treatment, and thorough follow-up.
Differential diagnosis
Table 12.3 presents conditions that are associated with mild elevations in cortisol, but are not considered Cushing’s syndrome per se. Women with physiologic elevations in cortisol may present with nonspecific features of a true Cushing’s syndrome – such as obesity, diabetes, and depression. Along with biochemical evidence of hypercortisolemia, it becomes very difficult to distinguish these patients from those with mild Cushing’s syndrome. For example, a depressed patient may present with weight gain, menstrual irregularities, reduced libido, mild hirsutism, and a urinary cortisol above the upper limit of normal. The clinician is then left to determine whether the depression led to the excess cortisol, or the excess cortisol led to the depression. Physiologic hypercortisolism is thought to result from over-activation of the HPA axis in response to a condition or stressor. It should resolve once the condition is reversed (i.e., when the depression is treated). It is also paramount to remember that while these conditions are relatively common in women, true Cushing’s syndrome is rare.
May present with clinical features of Cushing’s syndrome | Unlikely to present with clinical features of Cushing’s syndrome |
|---|---|
Pregnancy | Physical stress |
Depression/psychiatric conditions | Malnutrition/anorexia nervosa |
Alcoholism | Intense chronic exercise |
Glucocorticoid resistance | Hypothalamic amenorrhea |
Morbid obesity | Corticosteroid-binding globulin (CBG) excess |
Diabetes mellitus (poorly controlled) |
Diagnosis
The diagnosis of Cushing’s syndrome begins with a thorough history and physical searching for the clinical features outlined in Table 12.2 [29]. A comprehensive drug history helps investigate the possibility of iatrogenic Cushing’s syndrome. Glucocorticoids are found in many preparations (oral, rectal, inhaled, topical, injected) and may be found in many over-the-counter skin creams, bleaching agents, and herbal medications [25]. It is prudent to recognize that iatrogenic Cushing’s syndrome, as it is most common, can be easily reversed with withdrawal of the drug, and saves the patient from unnecessary testing.
Endogenous Cushing’s syndrome, on the other hand, is rare and the diagnostic workup is complex, expensive, and taxing. Therefore, the clinician must have a high index of suspicion before recommending such testing. The non-discriminate screening of patients with relatively common conditions such as diabetes mellitus, obesity, and depression is not recommended, owing to a high false-positive rate [25,29]. The current recommendation is to screen the following patient populations: [25]
1. Patients with features that are unusual for age, such as osteoporosis and hypertension in a young woman
2. Patients with multiple and progressive features, specifically those features which are more discriminatory (Table 12.2)
3. Children with decreasing height percentile and increasing weight
4. Patients with incidentally discovered adrenal adenomas.
Patients with a family history of Carney complex or MEN1 should undergo surveillance screening for the development of Cushing’s syndrome.
An algorithm for the diagnosis in a woman who is suspected of having Cushing’s syndrome is presented in Figure 12.3.
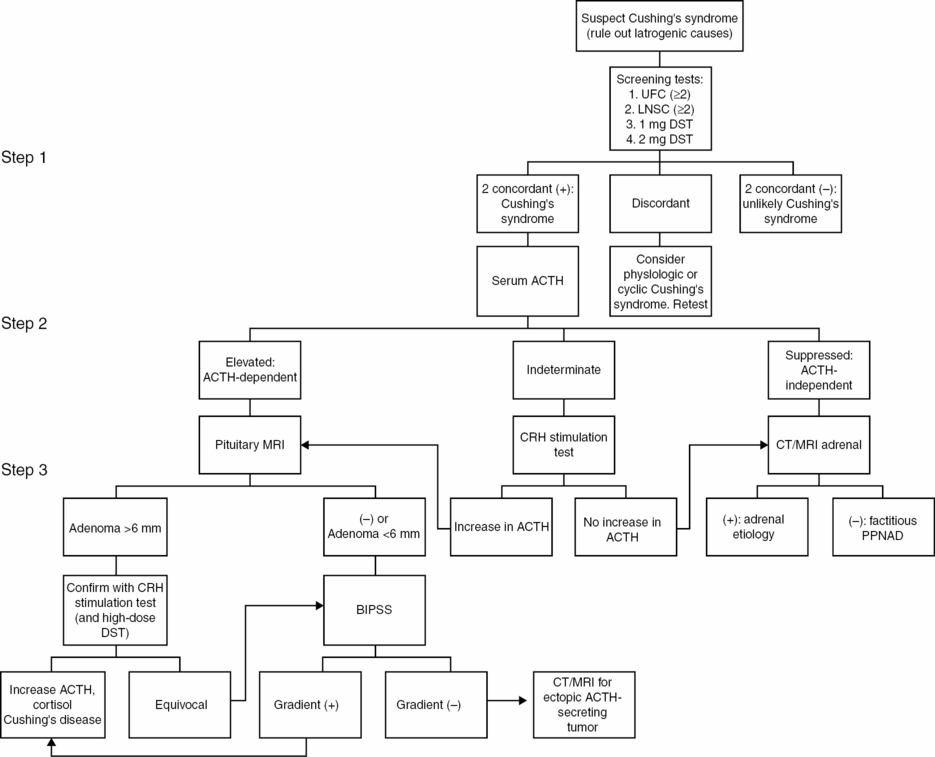
Biochemical testing
The diagnosis of Cushing’s syndrome is established with the following sequential steps:
1. Establishing the presence of hypercortisolism
2. Determining ACTH dependence vs. independence
3. Determining the source of ACTH in ACTH-dependent Cushing’s syndrome.
Step 1. Establishing the presence of hypercortisolism
Hypercortisolism is established with one of the following first-line tests [25]:
24-hour urine free cortisol (≥2)
Late-night salivary cortisol (≥2)
1 mg overnight dexamethasone suppression test
2 mg/day 48-hour low-dose dexamethasone suppression test.
There are two additional second-line tests used for specific situations:
2 mg/day 48-hour low-dose dexamethasone suppression test with CRH
midnight serum cortisol.
Table 12.4 provides a summary of the screening tests. They are highly sensitive and, with the exception of midnight serum cortisol, can be performed in an outpatient setting. The difficulty in the first step is in differentiating between true Cushing’s syndrome and other causes of hypercortisolemia, presented in Table 12.3. Therefore, an abnormal result from a first-line test, or a high clinical suspicion with a normal result, should prompt retesting with a different first- or second-line test. If the results are concordantly positive, Cushing’s syndrome is confirmed and further testing to elucidate the cause is indicated (step 2). If the results are concordantly negative, Cushing’s syndrome is unlikely and no further evaluation is necessary. If the results are discordant, and there is still a high clinical suspicion of a cyclical disease, then instructing the patient to collect a urinary free cortisol or late-night salivary cortisol, when symptoms arise, may be beneficial.
Test | Procedure | Measure | Diagnostic cut-off | Sensitivity (%) | Specificity (%) | False positives | False negatives |
|---|---|---|---|---|---|---|---|
24 -hour urine free cortisol | Collect voids over 24 h | Cortisol, creatinine | Assay upper limit of normal; ~80 µg/24 h | 91–96 | 91 | High fluid intake (>5 L/day) Exogenous steroids, carbamazepine, digoxin, fenofibrate | Renal failure Cyclic Cushing’s Mild Cushing’s |
Late-night salivary cortisol | Collect saliva sample between 11 p.m. and 12 a.m. | Salivary cortisol | 145 ng/dL | 92 | 96 | Ill or depressed patients Shift-workers Chewing tobacco, cigarette smoking Contamination/user error | Cyclic Cushing’s |
1 mg DST | 1 mg dexamethasone between 11 p.m. and 12 a.m. | Serum cortisol between 8 and 9 a.m. | 1.8 µg/dL | 98–100 | 80 | Medications inducing CYP 3A4 system Estrogens/pregnancy Decreased dexamethasone absorption Alcohol | Medications inhibiting CYP 3A4 system Liver and/or renal failure |
2 mg DST | 0.5 mg dexamethasone administered q6h starting at 9 a.m. for 48 h | Serum cortisol at 9 a.m. 6 h after last dose | 1.8 µg/dL | 96 | 79 | ||
2 mg DST with CRH | 2 mg DST followed by IV 1 µg/kg CRH 2 h after last dose | Serum cortisol 15 min after CRH | 1.4 µg/dL | 98 | 70 | ||
Midnight serum cortisol | Venous sampling from indwelling catheter at 12 a.m. | Serum cortisol | 1.8 µg/dL | 99–100 | 20 | Stress from hospitalization Ill or depressed patients | Cyclic Cushing’s |
7.5 µg/dL | 91–96 | 88 |
None of the tests for diagnosing Cushing’s syndrome are perfect, and a wide range of diagnostic thresholds have been proposed – each with different discriminatory powers, on different patient populations, and with different assays. Likely, this reflects that Cushing’s syndrome exists on a wide spectrum. Regardless, meta-analysis has shown a similar diagnostic accuracy for urine free cortisol, late-night salivary cortisol, and 1 mg overnight dexamethasone suppression test, as well as various combined strategies [39].
24-hour urinary free cortisol (UFC)
Cortisol circulates both freely and bound to corticosteroid-binding globulin (CBG).
Free cortisol is filtered by the kidneys and excreted into the urine. When measured over a 24-hour period, it provides a good indication of cortisol levels. Free cortisol is not affected by conditions that alter the level of CBG, such as estrogen. Plasma cortisol, on the other hand, measures both bound and free cortisol, and can be falsely elevated in patients on oral contraceptives, for example.
Patients are instructed to discard the first morning void on the first day and to collect subsequent voids throughout the day and night, including the first morning void on the second day. The urine should be kept refrigerated [25].
A value above the assay’s upper limit of normal (~80 µg/day) should prompt further testing. At leasttwo tests should be performed, as values can be variable. However, if several are normal, there is a low probability of Cushing’s syndrome. Mild elevations in UFC can be seen with physiological hypercortisolemia. Pregnancy, owing to estrogen, may present with UFC values up to three times normal. UFC values fourfold greater than the upper limit of normal, however, are virtually diagnostic of Cushing’s syndrome [29].
In clinical practice, the accuracy of the test is often limited by the adequacy of collection. Expressing free cortisol over urinary creatinine may help verify an adequate urine sample. Further, patients with renal failure and a creatinine clearance less than 60 mL/min may receive a falsely low result. Excessive amounts of fluids (>5 L/day) may falsely increase urinary cortisol. Finally, certain drugs – such as carbamazepine, digoxin, and fenofibrate – may co-elute with cortisol and falsely elevate urinary cortisol [25].
Late-night salivary cortisol
Late-night salivary cortisol is a simple and convenient non-invasive screening test.
Normally, cortisol rises in the early morning, peaks during mid-morning, and then falls to its nadir around midnight. Patients with Cushing’s syndrome lose their rhythmic cortisol fluctuation and fail to demonstrate depressed cortisol levels in the late evening. Salivary cortisol concentrations are an accurate representation of the free serum cortisol, independent of the salivary flow rate, and show high concordance with UFC in patients with Cushing’s syndrome [40,41].
The main advantage of testing salivary cortisol is in its ease of collection in the outpatient setting. Patients are instructed to collect saliva samples (by drooling into a plastic bag or chewing on a salivette for 1–2 minutes) between 11 p.m. and 12 a.m. on two occasions. The samples are stable at room temperature for up to a week, allowing patients to mail them to the lab. It can also be particularly useful if there is a need for repeated testing, an advantage when the presumed diagnosis is a cyclical Cushing’s syndrome.
Most normal patients have a late-night salivary cortisol concentration of less than 145 ng/dL by ELISA and mass spectrometry (LC-MS/MS) [25,42]. Patients should collect saliva while relaxed, as spuriously elevated values can result if patients are stimulated at the time of testing. Further, patients with abnormal circadian rhythms, such as shift workers or those in critical condition, may not be good candidates for testing. Patients should also be particularly careful to avoid contamination with steroid-containing lotions and gels.
Dexamethasone suppression tests
Negative feedback from an exogenous glucocorticoid should suppress serum ACTH and cortisol in patients with a normally functioning HPA axis. Women with Cushing’s syndrome, however, lose their HPA axis feedback regulation and do not demonstrate the expected suppression after the administration of a low dose of dexamethasone. There are three versions of the dexamethasone suppression test: the 1 mg overnight dexamethasone suppression test, the 2 mg/day 48-hour low-dose dexamethasone suppression test, and the 2 mg/day 48-hour low-dose dexamethasone suppression test with CRH.
Abnormalities in dexamethasone absorption and metabolism can produce false results. A false negative may result when dexamethasone clearance is slowed, either by hepatic or renal failure, or by drugs that inhibit the hepatic CYP 3A4 enzymes: aprepitant/fosaprepitant, itraconazole, ritonavir, fluoxetine, diltiazem, and cimetidine. Conversely, a false positive may result when dexamethasone clearance is increased by drugs that induce the hepatic CYP 3A4 enzymes: phenobarbital, phenytoin, carbamazepine, primidone, rifampin, rifapentine, ethosuximide, pioglitazone, and alcohol [25]. If there is concern about dexamethasone clearance, the simultaneous measurement of a serum dexamethasone (>0.22 μg/dL) with serum cortisol may be constructive [25]. Additionally, women should withhold oral estrogen-containing drugs for 6 weeks before testing [43]. Oral estrogens, such as oral contraceptives, and pregnancy may increase circulating CBG, yielding a false positive result.
The 1 mg overnight dexamethasone suppression test
The patient is given 1 mg of dexamethasone between 11 p.m. and 12 midnight and serum cortisol is measured between 8 and 9 a.m. the following morning. A cut-off of 1.8 µg/dL by radioimmunoassay achieves a high sensitivity, appropriate for screening [25]. However, the specificity is increased with an increasing serum cortisol suppression cut-off.
The 2 mg/day 48-hour low-dose dexamethasone suppression test
The 2 mg/day 48-hour low-dose dexamethasone suppression test is similar to the 1 mg overnight suppression test but spread over two days. The patient is given 0.5 mg every 6 hours beginning at 9 a.m. on the first day (9 a.m., 3 p.m., 9 p.m., 3 a.m.). This is repeated on the second day. Serum cortisol is then measured at 9 a.m. on the third day, 6 hours after the last dose of dexamethasone.
This test was once thought to provide more accuracy in discriminating between Cushing’s syndrome and other conditions of hypercortisolism, when 24-hour urinary free cortisol may be falsely elevated. However, recent studies have shown similar performance to the 1 mg overnight dexamethasone suppression test [25,38]. Additionally, because it offers equivocal diagnostic acumen and is more cumbersome to perform, it has largely fallen out of favor.
The 2 mg/day 48-hour low-dose dexamethasone suppression test with CRH
The 2 mg/day 48-hour low-dose dexamethasone suppression test with CRH was developed to improve the sensitivity of the 2 mg/day 48-hour low-dose dexamethasone suppression test in discriminating between true Cushing’s syndrome and states of physiological hypercortisolemia. A small population of patients with Cushing’s syndrome suppress cortisol in response to dexamethasone. Administration of CRH should increase ACTH and cortisol in those patients with Cushing’s syndrome only, whereas patients with physiological hypercortisolemia often have a blunted response to the administration of CRH.
The test is carried out similarly to the 2 mg low-dose dexamethasone suppression test, but 1 µg/kg CRH is administered intravenously (IV) 2 hours after the last dose of dexamethasone. Dexamethasone is measured at the time of CRH administration and cortisol is measured 15 minutes later. Although initially shown to yield higher sensitivity and specificity than the 2 mg/day 48-hour low-dose dexamethasone suppression test, more recent studies fail to show improved specificity [38]. It may be useful in patients with questionable urine cortisol levels [25].
Midnight serum cortisol
Similar to the late-night salivary cortisol test, the midnight serum cortisol test is based on the premise that women with Cushing’s syndrome lose their rhythmic cortisol fluctuation and therefore their nocturnal nadir. The patient is admitted to the hospital and serum cortisol is measured at midnight through an indwelling catheter. The patient is often kept for 48 hours to avoid false elevations from the initial stress of hospitalization. A serum cortisol greater than 1.8 µg/dL in a sleeping patient is highly sensitive [36]. Its greatest utility may be to exclude Cushing’s syndrome when a patient produces an elevated UFC and fails to suppress cortisol with dexamethasone testing, but there is a low clinical suspicion [25]. Specificity is increased if the cut-off is raised to 7.5 µg/dL. If the patient is awake, a serum cortisol greater than 7.5 µg/dL is highly sensitive, but less so than in a sleeping patient. Because of the cost and inconvenience associated with inpatient admission, serum cortisol is generally not used as an initial test.
Step 2. Determining ACTH dependence vs. independence
A morning serum ACTH at 9 a.m. will help determine the cause of an established Cushing’s syndrome. Blood is collected at 9 a.m. in a prechilled EDTA tube, placed in an ice water bath, and delivered to the lab quickly, as ACTH is degraded by plasma proteases. A serum ACTH depressed below 10 pg/mL is indicative of an ACTH-independent Cushing’s syndrome [29]. The next step is thin-section computed tomography (CT) or magnetic resonance imaging (MRI) of the adrenal glands to search for an adrenal etiology (adrenocortical adenoma, carcinoma, or ACTH-independent macronodular adrenal hyperplasia). If imaging fails to reveal the etiology, then consider PPNAD (either sporadic or part of the Carney Complex) or that the patient is surreptitiously using glucocorticoids.
A serum ACTH elevated above 20 pg/dL suggests an ACTH-dependent cause of Cushing’s syndrome [29]. The next step is a gadolinium-enhanced T1-weighted pituitary MRI and biochemical tests to determine the source of ACTH (step 3). For indeterminate tests (serum ACTH 10–20 pg/dL), a CRH stimulation test may be fruitful.
Step 3. Determining the source of ACTH in ACTH-dependent Cushing’s syndrome
It is important for treatment purposes to make the distinction between pituitary and ectopic ACTH-secreting tumors, although this is not always possible in clinical practice. Determining the source of ACTH in an ACTH-dependent etiology is often the most challenging aspect in the diagnosis of Cushing’s syndrome.
Clinically, patients with either etiology may be virtually indistinguishable. Imaging, although invaluable, fails to visualize a pituitary adenoma in roughly 40% of patients with Cushing’s disease [44]. Further, there is a possibility that a pituitary “incidentaloma” may be mistaken for an ACTH-secreting pituitary adenoma, when in fact, the patient has an ectopic ACTH-secreting tumor. Nearly 10% of the population have incidental pituitary tumors visualized on MRI [44]. This limits the reliance on imaging alone.
Measuring serum ACTH and potassium may clue the clinician into one etiology. Ectopic ACTH-secreting tumors tend to have higher serum ACTH values than pituitary ACTH-secreting tumors, but serum ACTH values are rarely able to definitively distinguish between the two etiologies [19]. Hypokalemia is common in patients with ectopic ACTH production. Excessive cortisol saturates 11β-hydroxysteroid dehydrogenase and interacts with the mineralocorticoid receptor in the renal tubules. This, however, is more often a reflection of high cortisol levels than specifically ectopic ACTH production. Notably, 10% of patients with Cushing’s disease may also have hypokalemia [19].
Dynamic testing is frequently used to determine a pituitary versus non-pituitary cause of Cushing’s syndrome. A summary of these tests is provided in Table 12.5. Testing can be non-invasive (high-dose dexamethasone suppression test, CRH stimulation test) or invasive (inferior petrosal sinus sampling). The clinical utility of each test is judged against the pretest probability that 85–90% of women with ACTH-dependent Cushing’s syndrome will have Cushing’s disease [19].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


