and Fatemeh Ghane Sharbaf2
(1)
Department of Pediatrics Section of Nephrology, Rush University Medical Center, Chicago, IL, USA
(2)
Department of Pediatrics Section of Nephrology, Mashhad University of Medical Sciences, Mashhad, Iran
CRRT Technical Considerations
The CRRT prescription requires the selection of a properly functioning vascular access, appropriate catheter size, and low extracorporeal line sets and hemofilter as well as the purpose of treatment and availability of work team including experienced nursing staff, dietitian, pharmacist, and technical support. If one were to suggest a standard prescription, then a blood flow rate (BFR) for CVVH would be in the range of 4–6 mL/kg/min trying to keep a venous return pressure of less than 200 mmHg [1–7]. Further there is no absolute data to date on the rate of replacement fluid or dialysate fluid. Traditionally, we have used a rate of 2000 mL/1.73 m2/h for this which allows us to compare pediatric data based on body surface area to adult data [1–7]. Thus, in a 10-kg child with a 0.5-m2 body surface area, the dialysate or replacement fluid rate prescribed would be roughly 700 mL/h.
Factors affecting hemodynamics include excessive ultrafiltration and inadequate replacement. Vasopressor agents including epinephrine, norepinephrine, and dobutamine all have in common small molecular weight and minimal protein binding and are rapidly removed by plasma clearance.
Ultrafiltration Rates (Removal Rates)
Each dialyzer has a specified ultrafiltration coefficient that is a measure of fluid that will pass from the membrane in 1 h. Dividing the fluid removal desired by hours of treatment gives the ultrafiltration rate. The amount ultrafiltered depends on transmembrane pressure between the blood and dialysate compartment.
The rate of ultrafiltration depends on the patient’s hemodynamic status. In children, a dose ranging from 35 to 40 mL/kg/h or 2 to 3 L/1.73 m2 of either connective or diffusive clearance would provide adequate urea clearance [1, 4, 8]. It is recommended to start ultrafiltration with zero and slowly increase to 1–2 mL/kg/h or 0.5–3 L/1.73 m2/h until fluid balance goal is achieved [1, 4, 5, 8].
Calculating the Desired Patient Fluid Removal Rate
The CRRT machine software automatically calculates the ultrafiltration rate needed to achieve the patient fluid removal rate (FRR ). The machine “control unit software,” however, does not measure or account for non-CRRT sources of patient fluid intake (such as hyperalimentation and blood or drug infusion) or fluid output (such as urine and wound drainage). It also does not account for anticoagulant solution infused for CRRT modality anticoagulant syringe pump. The operator must account for those sources when calculating the patient FRR, as well as when calculating the patient’s input/output totals.
Blood Flow Rates
A minimum of 30–50 mL/min blood flow is required to minimize access and filter clotting. The maximum BFR is 400 mL/min/1.73 m2 or 10–12 mL/kg/min in neonates and infants, 4–6 mL/kg/min in children, and 2–4 mL/kg/min in adolescents (Table 4.1).
Table 4.1
Choosing blood flow rate for pediatric CRRT
Body weight (kg) | <10 | 11–20 | 21–50 kg | >50 |
Blood flow rate (mL/min) | 24–50 | 80–100 | 100–150 | 150–180 |
The BFR generated by the PRISMA machine ranges from 5 to 10 mL/kg/min (up to 180 mL/min), which will allow adequate flow through the circuit [1, 4, 5, 8]. We use a minimum of 50 mL/min for newborn infants. The venous pressure should be preferably no higher than 250 mmHg. Table 4.1 demonstrates the recommended BFR during CRRT for pediatric patients.
Dialysate/Replacement Solution Flow Rates
The ultrafiltration rate/plasma flow rate (BFR × (1-Hct) ratio should be <0.35–0.4 in order to avoid filter clotting. Dialysate or effluent flow rates ranging from 20 to 35 mL/min/m2 (~2000 mL/h/1.73 m2) are usually adequate (adult data). The standard dialysate flow rate is 40–60 mL/kg/h or 2.5 L/h/1.73 m2 [1, 4, 8].
All CRRT techniques other than SCUF require the use of dialysate or replacement solution to compensate for the effluent fluid and electrolyte removal. Optimal dialysate or replacement solution approximates normal plasma water composition, replacing electrolytes and minerals in physiologic concentrations without replacing the metabolic solutes, which accumulate in AKI. The composition of these solutions can be varied extensively to achieve specific metabolic goals (e.g., bicarbonate-based solutions can be used to correct acidemia and the electrolyte content can be altered to correct electrolyte imbalance) [8–14].
When citrate anticoagulation is used in CRRT, modifications are necessary in both the replacement fluid and dialysate [15–19]. Citrate is metabolized to bicarbonate by the liver; therefore, buffer is not generally required in the dialysate [9, 20–26]. Similarly, dialysate used in citrate regional anticoagulation is generally hyponatremic to prevent hypernatremia, and it is recommended that fluids are calcium-free. Few commercially available calcium-free, bicarbonate-based CRRT fluids have been available until recently.
Lactate- and acetate-based solutions are no longer recommended for patients with multiorgan failure undergoing CRRT because of patients’ limited capacity to convert these buffers to bicarbonate [11, 27].
Bicarbonate-based solutions are the solution of choice for patients under CRRT [14]. Patients on CRRT tend to develop hypophosphatemia. However, a combination of bicarbonate, calcium, and phosphorus in the same bag may increase the risk of precipitation. To avoid this, phosphorus should be given to the patient in a line separate from the circuit. The bicarbonate concentration in the solution should be lowered to 22–25 mEq/L to avoid metabolic alkalosis, if a citrate anticoagulation protocol is used.
There is no absolute data to date on the rate of replacement fluid or dialysate fluid. A rate of 2 L/h/1.73 m2 is recommended for pediatrics CRRT (CVVH, CVVHD, CVVHDF).
Net Fluid Balance
Fluid balance should take into account the patient’s hemodynamics status, volume status, and fluid requirements. A suggested net ultrafiltration per hour is 2 L/1.73 m2/h [1, 4, 8]. Aggressive ultrafiltration should be avoided in hemodynamic unstable patients. In such setting, solute clearance can be achieved without ultrafiltration.
The system is very efficient; thus, too much fluid can be removed too quickly. This is the perfect time to maximize the total parenteral nutrition (TPN) of the patient. Further, in order to get maximum fluid shift with colloid infusion, the ultrafiltration rate for the colloid previously infused should be over twice as much as the colloid rate “in” (e.g., 200 mL of PRBCs infused over 2 h should be ultrafiltrated over 4 h).
The patient net FRR that should be set on the CRRT machine control unit may be calculated using the following formula:
![$$ \begin{array}{l}\mathrm{P}\mathrm{atient}\;\mathrm{F}\mathrm{R}\mathrm{R}\left(\mathrm{mL}/\mathrm{h}\right)\\ {}\mathrm{set}\;\mathrm{in}\;\mathrm{CRRT}\;\mathrm{machine}=[\mathrm{N}\mathrm{e}\mathrm{t}\;\mathrm{fluid}\;\mathrm{removal}\left(\mathrm{U}\mathrm{D}\right)\mathrm{rate}\left(\mathrm{mL}/\mathrm{h}\right)+\mathrm{fluid}\;\mathrm{in}\mathrm{t}\mathrm{ake}\\ {}\kern5.16em \left(\mathrm{I}\mathrm{V},\mathrm{T}\mathrm{P}\mathrm{N},\mathrm{citrate},\mathrm{calcium}\;\mathrm{in}\mathrm{fusion}\;\mathrm{rates}\left(\mathrm{mL}/\mathrm{h}\right)\right]\\ {}\kern5.16em -\left[\mathrm{N}\mathrm{on}-\mathrm{CRRT}\ 0.5em\mathrm{outputs}\left(\mathrm{mL}/\mathrm{h}\right)\mathrm{urine},\ 0.5em\mathrm{e}\mathrm{t}\mathrm{c}.\right]\end{array} $$](/wp-content/uploads/2016/07/A370551_1_En_4_Chapter_Equa.gif) The patient net FRR must be adjusted if the weight loss prescribed by the physician is changed or if the patient’s non-PRISMA fluid inputs or outputs change.
The patient net FRR must be adjusted if the weight loss prescribed by the physician is changed or if the patient’s non-PRISMA fluid inputs or outputs change.
![$$ \begin{array}{l}\mathrm{P}\mathrm{atient}\;\mathrm{F}\mathrm{R}\mathrm{R}\left(\mathrm{mL}/\mathrm{h}\right)\\ {}\mathrm{set}\;\mathrm{in}\;\mathrm{CRRT}\;\mathrm{machine}=[\mathrm{N}\mathrm{e}\mathrm{t}\;\mathrm{fluid}\;\mathrm{removal}\left(\mathrm{U}\mathrm{D}\right)\mathrm{rate}\left(\mathrm{mL}/\mathrm{h}\right)+\mathrm{fluid}\;\mathrm{in}\mathrm{t}\mathrm{ake}\\ {}\kern5.16em \left(\mathrm{I}\mathrm{V},\mathrm{T}\mathrm{P}\mathrm{N},\mathrm{citrate},\mathrm{calcium}\;\mathrm{in}\mathrm{fusion}\;\mathrm{rates}\left(\mathrm{mL}/\mathrm{h}\right)\right]\\ {}\kern5.16em -\left[\mathrm{N}\mathrm{on}-\mathrm{CRRT}\ 0.5em\mathrm{outputs}\left(\mathrm{mL}/\mathrm{h}\right)\mathrm{urine},\ 0.5em\mathrm{e}\mathrm{t}\mathrm{c}.\right]\end{array} $$](/wp-content/uploads/2016/07/A370551_1_En_4_Chapter_Equa.gif)
Circuit Priming
Priming a hemofilter circuit is recommended when circuit volume is >10 % of patient’s intravascular blood volume. Heparinized 0.9 % saline (5000 U/L) is commonly used for most patients. Smaller patients require blood priming to prevent hypotension particularly when the circuit volume is >10–15 % patient blood volume.
Risks of using blood for prime include bradykinin release syndrome (bronchospasm, hypotension, mucosal congestion), hyperkalemia, hyponatremia, and hypocalcemia [28, 29]. The pH of blood coming in contact with electronegatively charged membranes during prime elicits bradykinin response especially in patients <10 kg (standard blood from a blood bank has a pH of 6.4). The lower the pH, the more bradykinin released from blood. Saline (pH 5.0) does not elicit a bradykinin response because it does not contain bradykinin or other blood substances. CRRT membrane AN-69 is highly biocompatible with low thrombogenicity and electronegative surface. Its use has been associated with a bradykinin release syndrome particularly in patients on ACE inhibitors or in acidotic patients. Blood-banked blood has a pH of 6.4 with calcium being essentially zero. This has been shown to cause the bradykinin release syndrome that clinically appears to be anaphylaxis associated with acute hypotension, tachycardia, and a drop in the CVP. This is immediately reversible by removing the system and may be avoided by buffering the blood or by post-hemofiltration blood transfusion [28, 29].
The blood buffer solution can be prepared by mixing 43 mL of blood-banked blood with 7 mL of THAM, 50 mL of a solution of 150 mEq of NaHCO3 in 1000 mL of water, 45 mg of CaCl2, and 2 U/mL of heparin. The final pH of this is 7.41 and the Ca++ is 1.0 mmol/L. 90 mL of this combination may be used for blood priming and may prevent bradykinin release syndrome. Following blood transfusion, monitor the child’s pH and give sufficient NaHCO3 to the child to bring the pH to > 7.35.
Anticoagulation
Many children who require CRRT will need anticoagulation to prevent filter membrane clotting and blood loss [15–19, 30]. Insufficient anticoagulation may affect filter longevity and decreases dialyzer efficiency and performance. On the other hand, excessive anticoagulation may result in bleeding complications or thrombocytopenia [31–35]. The adult data on the use of regional citrate anticoagulation during CRRT show a decreased risk of bleeding and at the least equivalent circuit survival as compared to heparin. Current pediatric and adult studies support regional citrate anticoagulation as an effective alternative to systemic heparin anticoagulation in most patient populations.
Heparin-Free CRRT
CRRT can be performed without anticoagulation, especially in children with high risk of bleeding. Children with activated clotting time (ACT) >200 s, low platelet count, and liver failure can also be treated without the use of anticoagulation [33, 35].
Multiple organ dysfunction syndrome classically occurs in patients who also suffer from abnormal clotting parameters. Usually these patients ar e given ample amount of platelet infusions and coagulation factors. This excessive amount of volume adds to greater need for ultrafiltration and greater risk for clotting.
In heparin-free CRRT, blood flows are maintained above 5–10 mL/min, and saline flushes should be administered (40–45 mL) every 15–30 min into the arterial limb of the circuit to maintain circuit patency.
Heparin Protocol
Systemic administration of heparin with protamine infusion back to the patient should be avoided because it does not improve circuit life and may cause coagulopathy in the patient [15–19, 30]. Heparin is generally administered as a bolus (10–20 U/kg), followed by continuous infusion (10–20 U/kg/h) into the arterial limb of the CRRT circuit (pre-filter) to maintain ACT between 180 and 200 s or partial thromboplastin time of two times normal. The infusion rate of heparin is adjusted to the keep the ACT at target level between 170 and 220 s (Table 4.2). ACT should be checked every 1–4 h on the “venous” side of the hemofilter, meaning after the hemofilter (post-filter). Adjust post-filter ACT between 180 and 200 s. If ACT is >150 or <180 s, then no load is necessary; just start the drip. If ACT is >200, no heparin is necessary and recheck in 30 min for 2–3 h. Once an established ACT pattern is identified, then check ACT q4 h (Table 4.2).
Table 4.2
Heparin dosing adjustment during CRRTa
If the ACT is | Make this change |
|---|---|
170–220 | No change |
>220 | HOLD heparin for 1 h, then decrease the infusion by 10 % less/hour, and recheck ACT in an hour |
<170 | Administer bolus of 10 U/kg, then increase the infusion by 10 %, andrecheck ACT in an hour |
Heparin Dose Adjustment
Determine heparin concentration as follows:
Patient weight | Heparin concentrationa (U/mL) |
|---|---|
<10 kg | 40 |
11–25 kg | 100 |
16–60 kg | 250 |
>60 kg | 500 |
aPRISMA heparin rate must be ≥0.5 mL/h
Initial bolus is 20 U/kg (administer pre-filter). Subsequent hourly continuous infusion (10–20 U/kg/h) is given to maintain APTT of 1.5–2.5 times the control value (25–35 s).
Adjust heparin infusion rate according to APTT as follows:
APTT (s) | Heparin infusion rate |
|---|---|
50–80 | No change |
<50 | Administer bolus of 10 U/kg, increase by 10 %, and recheck APPT in 1 h |
>80 | Hold heparin infusion by ½ h, decrease by 10 %, and recheck APPT in 1 h |
Patients who develop heparin-induced thrombocytopenia (HIT) frequently need further anticoagulation to treat an ongoing thromboembolic problem or to prevent one. Orgaran, a low-molecular-weight (LMW) glycosaminoglycuronan, has shown a low frequency (10 %) of cross-reactivity in vitro with sera containing the HIT antibody, in contrast to the much higher frequency of cross-reactivity (approximately 80 %) shown by the LMW heparins [33, 35]. This study summarizes the results of intravenous or subcutaneous Orgaran treatment in 57 patients, in whom the diagnosis of HIT was reasonably confirmed by exclusion of other causes of thrombocytopenia and by objective tests. The presenting indications for Orgaran were continuous veno-venous hemofiltration and hemodialysis (n = 21), thromboembolism treatment (n = 23), thromboembolism prophylaxis (n = 10), and anticoagulation for coronary artery bypass graft (n = 4), peripheral bypass graft surgery, and plasmapheresis (n = 1 each). The results showed Orgaran to be a safe, well-tolerated, effective (successful treatment in over 90 % of patients) anticoagulant in patients with a high thrombotic and/or bleeding risk even if critically ill and requiring hemofiltration.
Low-molecular-weight heparin has higher anti-x and is a more reliable anticoagulant than heparin. Its pharmacokinetics is more predictable because of less plasma protein binding. However, there are no significant differences between the low-molecular-weight heparin and unfractionated heparin in reduced risk of bleeding, thrombocytopenia, or filter life. Low-molecular-weight heparin will accumulate in patients with AKI and should not be used in patients with AKI, as the drug will accumulate during CRRT. If used, follow anti-factor Xa levels and reduce the dosing interval.
Citrate Dextrose (ACD)
Clotting is a calcium-dependent mechanism. Removal of calcium from the blood will inhibit clotting. Adding citrate to blood will bind the ionized calcium in the blood thus inhibiting clotting. The use of citrate anticoagulation has become increasingly popular [10, 18, 20–22, 24–26, 34, 36–39]. Unlike heparin, citrate has no effect upon patient bleeding, and it is easy to administer and monitor with calcium assay and is cost effective. Regional citrate anticoagulation is performed using a continuous infusion through the arterial limb of the circuit. The citrate chelates free Ca++ and the citrate-calcium complex is removed by CRRT, which inhibits the coagulation cascade. Therefore, plasma Ca++ levels should be maintained with the use of a continuous Ca++ infusion.
Citrate anticoagulation requires a separate central line (for calcium infusion). The infusion rate of citrate is adjusted to maintain target blood ACT level (Table 4.3). Regional citrate infusion requires the use of a commercially available solution and frequent monitoring of plasma Ca++ level (Table 4.4).
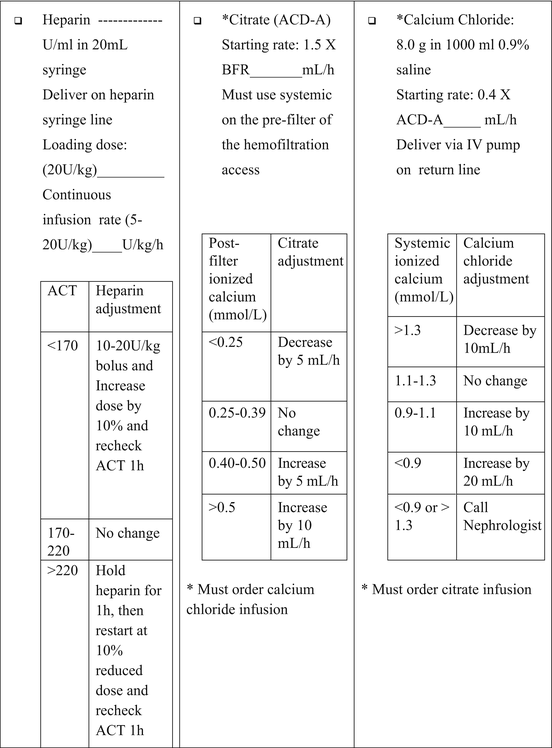
Table 4.3
Anticoagulation protocola

Table 4.4
Titrate the calcium infusion according to the calcium sliding scale
Patient ionized Ca++ (mmol/L) | Calcium infusion adjustment | |
|---|---|---|
>20 kg | <20 kg | |
>1.3 | ↓ rate by 10 mL/h | ↓ rate by 5 mL/h |
1.1–1.3 (optimum range) | No adjustment | |
0.9–1.1 | ↑ rate by 10 mL/h | ↑ rate by 5 mL/h |
<0.9 | ↑ rate by 20 mL/h | ↑ rate by 10 mL/h |
Citrate Protocol for CVVH (PRISMA)
1.
Prime tin CVVHDF mode using dialysate (bicarbonate based without calcium) and replacement solutions.
2.
Obtain from pharmacy 1000 mL anticoagulant citrate dextrose (ACD) solution and connect the ACD to a regular iv pump and attach it to the “pre-hemofilter arterial” 3-way stopcock.
3.
Infuse ACD rate (mL/min) at 1.5 × BFR (mL/min) (for instance, if PRISMA BFR is 100 mL/min, start ACD at 150 mL/h).
4.
In children less than 10 kg who are receiving blood transfusion, avoid the use of ACD for the first 15 min to prevent or retard the bradykinin release syndrome seen in some patients.
5.
Start the calcium chloride infusion (8 g in 1 L 0.9 % saline) at 40 % of the ACD flow rate via the central line other than the dialysis access (for instance, if the citrate rate is 150 mL/h, then the calcium chloride rate will be 60 mL/h) (Tables 4.4 and 4.5).
Table 4.5
Anticoagulant agents for CRRTa
Anticoagulant agent | Filter prime | Initial dose | Maintenance dose | Monitoring | Comments |
|---|---|---|---|---|---|
0.9 % saline solution | 2 L saline | 150–250 mL pre-filter | 100–250 mL/h pre-filter | Visual check | No anticoagulant used |
Heparin | 2500–10,000 U/2 L saline | 5–10 U/kg | 3–12 U/kg/h | ACT 200–250; PTT 1.5–2.0 × normal | Simple and easy to use |
LMW heparin | 2 L saline | 40 mg | 10–40 mg/6 h | Factor Xa levels; maintained between 0.1 and 0.41 U/mL | Lower risk of bleeding |
Regional heparin | 2500 U/2 L saline | 5–10 U/kg | 31–12 U/kg/h; +protamine post-filter | PTT: post-filter ACT 200–250 | Lower risk of bleeding |
Regional citrate | 2 L saline | 4 % trisodium citrate 150–180 mL/h | 100–180 mL/h 3–7 % of BFR, Ca replaced by central line 4–8 ng/kg/min | ACT: 200–250; maintain ionized calcium 1.0–1.2 nmol/L | No bleeding; no thrombocytopenia; better filter efficacy, longevity |
6.
Set flow rates in PRIMA as ordered.
7.
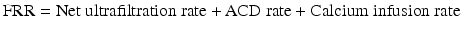
Calculate patient FRR by using the following equation:
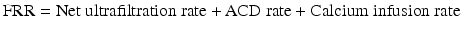
8.
Connect the CVVH (PRISMA circuit) to the dialysis catheter and press the start key.
9.
Draw blood 2 h after initiation of ACD infusion and every 6 h thereafter for post-hemofilter (the return line) for ionized calcium and from peripheral blood or from patient arterial line for electrolytes, BUN, creatinine, ionized calcium, phosphorus, and albumin.
12.
If serum bicarbonate falls below 35 mEq/L, add 0.9 % saline (200–400 mL/h) as replacement solution and decrease the dialysate rate by the same amount. This will give an acid load from the normal saline and lowers the bicarbonate from the bath at the same time.
13.
If the patient systemic blood ionized calcium falls below 0.75 mmol/L, stop ACD infusion for 1 h and resume infusion at 30 % of citrate flow rate. Also administer bolus of 10 mg/kg of calcium chloride and increase calcium infusion by 10 %.
14.
If the serum sodium is greater than 150 mEq/L, change replacement fluid to 0.45 % saline.
15.
If the filter clots, stop the ACD and calcium infusions and replace the filter.
Citrate is metabolized to bicarbonate by the liver; as a result, buffer is not generally required in the dialysate.
Citrate infusion is recommended in patients targeted for heparin-free CRRT and those requiring lower BFR. Filter life above 96 h is common with citrate anticoagulation and it does not cause HIT syndrome.
The disadvantages of using citrate anticoagulation include hypocalcemia, hypomagnesemia, hypernatremia, metabolic alkalosis, and citrate toxicity in patients with liver failure.
In children who receive “blood priming” and are on citrate anticoagulation, withhold the citrate for the first 10–15 min until hemodynamic stability is achieved. During this time, if needed, give 20 U/kg of heparin as one-time dose to anticoagulate the system until the citrate is begun. Citrate is acidic and may exacerbate the bradykinin release reaction.
The combination of bicarbonate-based solutions with bicarbonate concentrations >30 mEq/L and citrate anticoagulation often will result in metabolic alkalosis. Therefore, if citrate anticoagulation is used, it is preferred to use a replacement solution with bicarbonate level between 22 and 25 mEq/L as well as a zero calcium bath.
In summary, regional citrate anticoagulation is gaining popularity for CRRT in the critically ill patient, with either similar or longer CRRT circuit life compared to standard systemic anticoagulation with unfractionated or LMW heparins, but with reduced risk of hemorrhage and blood transfusion requirement. The dose of citrate needs to adjusted for blood flow, to achieve a pre-hemofilter/dialyzer ionized calcium target of <0.25 mmol/L, approximately corresponding to a citrate concentration of 4–6 mmol/L, for effective anticoagulation. Calcium is then reinfused to maintain a normal systemic ionized calcium concentration, and the citrate infusion adjusted according to the post-filter ionized calcium and the total systemic serum calcium. Patients can become alkalotic due to the metabolism of an increasing citrate load returning to the patient but also acidotic if citrate cannot be readily metabolized or the amount of citrate infused is too low. In addition, this may be compounded by nursing and/or fluid composition errors. However, by carefully monitoring ionized and total calcium, appropriate adjustments can be made to dialysate and/or replacement fluid rates and citrate and/or calcium infusion rates, to achieve acid–base targets. Although citrate is predominantly metabolized by the liver, many patients with liver disease, even those with cirrhosis, can often adequately metabolize the citrate load.
Prostacyclin
Prostacyclin (epoprostenol), a potent vasodilator and antithrombotic and antiplatelet agent, has been safely used to prevent clotting of the extracorporeal circuits either alone in patients with thrombocytopenia and/or increased risk of bleeding or in combination with heparin in a state of hypercoagulability [41].
Prostacyclin infusion pre-filter decreases bleeding risk without increasing platelet consumption. Systemic administration of prostacyclin does not prolong filter life during CVVH (www.pcrrt.com). Initial dose is 4 ng/kg/min (range 2–8 ng/kg/min). Monitor circuit life. If less than 48 h, increase sequentially by 2 ng/kg/min to max of 8 ng/kg/min. Closely monitor side effects (hypotension, facial flushing, hyperthermia, headache).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


