CHAPTER 15 Control of hypothalamic–pituitary–ovarian function
Anatomy of the Hypothalamic–Pituitary Axis
The adenohypophysis consists of:
Neural connections
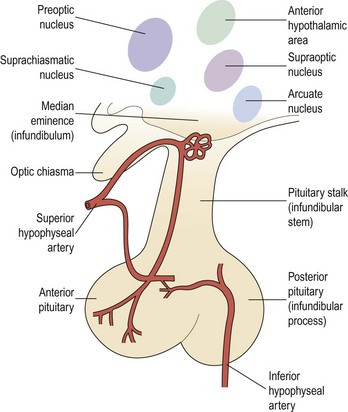
There are numerous and extensive neural pathways connecting the hypothalamus with the rest of the brain. The majority of afferent hypothalamic nerve fibres run in the lateral hypothalamic areas, whilst efferent pathways are more medially placed. One important efferent connection is the supraopticohypophyseal nerve tract carrying fibres from the supraoptic and paraventricular nuclei to the infundibular process of the pituitary, whilst other fibres carry hypothalamic-releasing or -inhibiting factors from the medial and basal parts of the hypothalamus to the anterior pituitary (Figure 15.2).
Gonadotrophin-releasing hormone (GnRH)-secreting neurones appear in the medial olfactory placode and enter the brain with the nervus terminalis, a cranial nerve that projects from the nose to the septal preoptic nuclei in the brain. By migration during embryogenesis, the cells, between 1000 and 3000 GnRH-producing neurones, predominantly settle in the arcuate nucleus of the hypothalamus (Schwanzel-Fukunda et al 1989). Failure of this migration has been shown to result in Kallmann’s syndrome; a disorder associated with an absence of GnRH secretion and a defect of smell-anosmia (a failure of both olfactory axonal and GnRH neuronal migration from the olfactory placode). A 5–7-fold increased frequency of this condition is found in males, indicating that an X-linked transmission is the most common, although autosomal-dominant and autosomal-recessive modes of transmission have also been established (Waldstreicher et al 1996).
The mutations responsible for this syndrome result in the failure to produce a protein, homologous to members of the fibronectin family, responsible for cell adhesion and protease inhibition that is necessary for migration of these neurones (Bick et al 1992). GnRH neurones have cilia — as do olfactory epithelial cells in the nose — and the olfactory origin and structural similarity of these cells suggest an evolution from reproduction controlled by pheromones.
Hypothalamic Regulation of Pituitary Secretion
Considerable efforts have been made in the past 25 years to identify, characterize and synthesize the substances thought to be produced in the neural elements of the infundibulum. Several substances which can either stimulate or suppress the rate of release of one or more hormones from the pituitary gland have been found in the infundibular complex. These can be classified into hypophysiotrophic, neurohypophyseal and pituitary peptide hormones and are listed in Table 15.1. Other substances will probably be added to this list in the future.
Table 15.1 Hypothalamic and pituitary hormones
| Hypothalamic hormones | |
| Gonadotrophin-releasing hormone | GnRH |
| Thyrotrophin-releasing hormone | TRH |
| Corticotrophin-releasing factor | CRF |
| Growth-hormone-releasing hormone | GHRH |
| Somatostatin | |
| Prolactin-inhibiting factor | PIF |
| Growth hormone secretagone receptor | Ghrelin |
| Posterior pituitary products | |
| Vasopressin | |
| Oxytocin | |
| Neurophysin I and II | |
| Anterior pituitary peptide hormones | |
| Adrenocorticotrophic hormone | ACTH |
| Prolactin | PRL |
| Luteinizing hormone | LH |
| Follicle-stimulating hormone | FSH |
| Growth hormone | GH |
| Thyroid-stimulating hormone | TSH |
GnRH
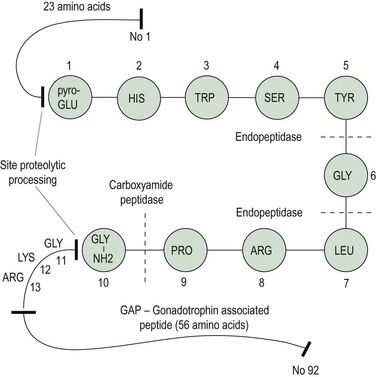
In 1971, Schally et al isolated pure preparations of porcine LH-releasing hormone (LHRH) from hypothalamic extracts. Subsequently, its structure was discovered and synthesis was achieved (Matsuo et al 1971a, b). The finding of FSH-releasing activity of this LHRH led to the hypothesis of a single hypothalamic-releasing hormone, GnRH, controlling secretion of both LH and FSH from the pituitary gland, with the suggestion that sex steroids might play a role in modulating the proportions of LH and FSH released. The amino acid sequence of GnRH is shown in Figure 15.3; it is a decapeptide secreted with a large pre- and postprecursor molecule.
GnRH neurone system
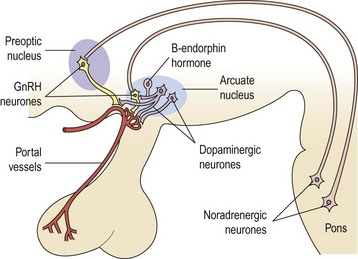
The GnRH neurone system has been mapped in detail using primarily immunocytochemical methods. The GnRH neurones are not grouped into specific nuclei but form a loose network in several anatomical divisions. However, GnRH neurone bodies are found principally in two areas: the preoptic anterior hypothalamic area and the tuberal hypothalamus, particularly the arcuate nucleus and periventricular nucleus. Axons from GnRH neurones project to many sites in the brain; the most distinct tract is from the medial basal hypothalamus to the median eminence, where extensive plexuses of boutons are found on the primary portal vessels. GnRH, then, has ready and direct access to the anterior pituitary gonadotroph cells via the portal capillary plexus (see Figure 15.2). There are also numerous projections of GnRH-secreting neurones to the limbic system and the circumventricular organ, other than the median eminence. The role of these connections is currently unknown, but they may connect with other cells or GnRH may bind to different receptors (type II) which may modulate sexual behaviour or sexual arousal.
Regulation of gonadotrophin secretion by GnRH
The GnRH gene sequence was first isolated by Seeburg and Adelman in 1984. The GnRH decapeptide is derived from the post-translational processing of a larger precursor molecule that has been termed ‘pre–pro-GnRH’. This appears to be a tripartite structure with a preceding 23-amino acid sequence joined to the decapeptide GnRH, which is then attached via a 3-amino acid sequence, glycine–lycine–arginine (GLY–LYS–ARG), to a 56-amino acid terminal peptide, which is termed the ‘gonadotrophin-associated peptide’ (GAP). The post-GnRH-decapeptide GLY–LYS–ARG 3-amino acid section is an important site for proteolytic processing. GAP itself is thought to have some prolactin-inhibiting properties (Figure 15.3). GnRH genes are encoded from a single gene located on the shorter arm of chromosome 8.
Using radioimmunoassays, GnRH has been demonstrated in the hypophyseal portal blood from a number of animal species (Carmel et al 1976). Electrical stimulation of the preoptic area of the brain in female rats on the day of pro-oestrus increases the GnRH concentration in portal blood, and the stimulus induces a marked release of LH from the anterior pituitary. In contrast, administration of antibodies against GnRH prevents this electrically stimulated LH release. These data provide evidence favouring a cause-and-effect relationship between GnRH release by the hypothalamus and LH release by the anterior pituitary.
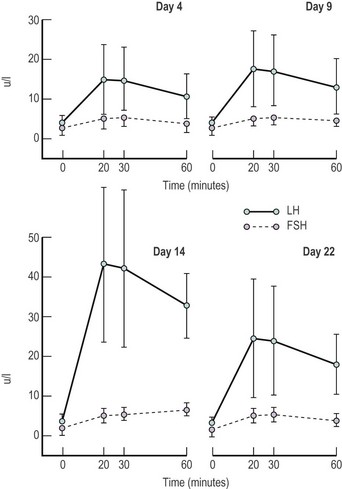
In the female, the magnitude of gonadotrophin release, particularly LH, in individuals varies with the stage of the menstrual cycle, being greatest in the preovulatory phase, less marked in the luteal phase and least in the follicular phase of any individual cycle (Yen et al 1972, Shaw et al 1974) (Figure 15.4).
Mechanism of action of GnRH on pituitary cells
There are three principal positive actions of GnRH on gonadotrophin secretion:
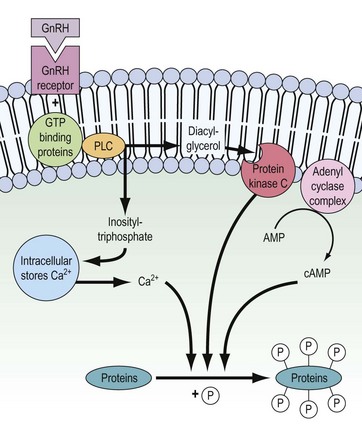
The binding of GnRH to its receptor induces a complex series of intracellular responses, which result in hormone secretion and biosynthesis of the α and β subunits of LH and FSH. In addition, dimerization of α and β subunits and the glycosylation processes are induced. The mechanism of action of GnRH is depicted in Figure 15.5. Within seconds of GnRH binding to and activating GnRH receptors on the pituitary gonadotrophs, intracellular free Ca2+ concentrations increase. This Ca2+ is initially mobilized from intracellular stores (e.g. endoplasmic reticulum) but also, to maintain sustained LH release, extracellular Ca2+ enters the gonadotroph through receptor-regulated voltage-dependent Ca2+ channels.
The initial mobilization of intracellular Ca2+ is induced by inositol triphosphate, released as a consequence of receptor activation of the membrane-bound phospholipase-C enzyme. Diacylglycerol is also released by the action of phospholipase-C and in turn activates the phosphorylating enzyme protein kinase C. The adenyl cyclase complex is also stimulated and cyclic adenosine monophosphate (cAMP) is generated. Ca2+, protein kinase C and cAMP then interact to stimulate release of stored LH and FSH and subsequent biosynthesis (for review, see Clayton 1989).
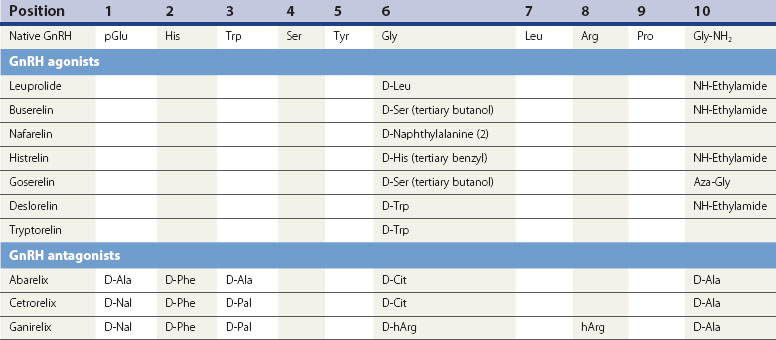
GnRH agonist analogues
The current generation of GnRH analogues in clinical use are predominantly agonists. GnRH is degraded by peptidases in the pituitary and hypothalamus which cleave the native decapeptide at the Gly6-Leu7 bond and at position 10. Substitution at position 6 with another amino acid renders the analogue less susceptible to enzymatic breakdown, resulting in a half-life 2.5–6 times longer than the native GnRH, whilst still allowing the analogue to bind to the pituitary gonadotroph GnRH receptor site. Several agonistic analogues are available for clinical use (see Table 15.2). Following an initial few days of increased secretion of LH and FSH after the first administration of a GnRH agonist, downregulation of the GnRH receptor is achieved with subsequently suppressed release of LH and FSH, and hence a reduced stimulus to the ovary resulting in failure of follicle growth and reduced ovarian steroidogenesis.