Population
Incidence per 10,000 women/year
General population of women <35 years old
1–5
Oral contraceptive pills (OCPs) users
3–10
Second generation progestin-containing OCPs
6
Drospirenone-containing OCPs
10
Pregnant women
5–20
Immediate postpartum women
45–65
Leiden mutation carriers
30–80
Leiden mutation carriers PLUS OCPs
50–100
Homozygous Leiden mutation carriers
400–800
Weight Gain
The only contraceptive method shown to be associated with weight gain is DMPA. One randomized trial of DMPA vs. placebo showed no increase in weight gain over 3 months [18]. However, another study found that 25% of DMPA-users gained more than 10% of their baseline weight [19]. However, it is unclear what causes the weight gain (e.g., increased appetite versus change in metabolism), and it is difficult to predict who will be affected. Some reports have found that higher baseline weight may predict greater weight gain while using DMPA; however, this finding is not consistently reported [20]. Data about weight gain while using the contraceptive implant is mixed, but the mean increase was less than 5 pounds over 2 years [21]. CHCs and IUDs have not been found to be associated with weight gain [22].
DMPA
Limited evidence suggests that among women with uncontrolled hypertension, those who used DMPA had a small increased risk for cardiovascular events compared to women using non-hormonal methods. However, these risks were significantly less than among CHC users [23]. Therefore, DMPA is listed as U.S. MEC 3 in women with uncontrolled hypertension, ischemic heart disease, multiple risk factors for cardiovascular disease, lupus with positive or unknown antiphospholipid antibody status or antiphospholipid syndrome, or current or past ischemic heart disease or stroke. However, risks of using DMPA must be weighed against the risks of pregnancy in women with uncontrolled hypertension or other comorbidity.
Breast Cancer
Because breast cancer is a hormonally sensitive tumor, there is concern that the prognosis for women with current or recent breast cancer might worsen with the use of estrogen or progestin-containing methods. Therefore, the use of any hormonal method of contraception is not recommended in women with current or past breast cancer (U.S. MEC 3 or 4). Given the lower systemic concentrations of progestin associated with the LNG-IUS, there is less concern related to this method, but the data are insufficient to make a definitive recommendation. Given the paucity of data, decision-making should be individualized, ideally within a multidisciplinary team. Current evidence does not suggest that any contraceptive method further increases the risk for breast cancer among women with either a family history of breast cancer or a known breast cancer susceptibility gene [24]. Therefore, no contraceptive method is restricted among these women (all methods U.S. MEC 1).
Depression
There is no increased risk of clinical depression in women using CHCs; in fact, Keyes and colleagues noted that CHC use may even be associated with lower rates of depressive symptoms among young women [25]. Women who report mood changes with the use of CHCs often experience this during the hormone-free periods, indicating that estrogen withdrawal may be responsible. In these cases, continuous or extended use formulations may be helpful. If mood changes occur during use of active pills, switching to a lower dose estrogen is reasonable. Data evaluating the impact of DMPA on depression are limited and conflicting, with few studies showing a small increased likelihood of depression in DMPA users and most others showing no association. However, it is reasonable to discontinue any method if a woman reports worsening depression with use. Given the risks of uncontrolled depression in pregnancy, an alternative method of contraception is essential, as well as ensuring adequate mental healthcare.
Intrauterine Devices (IUDs )
IUDs are first line-contraceptive options, and there are few contraindications to their use. Medical conditions of concern with the use of IUDs are limited, and mostly relate to infections and abnormalities of the genital tract. Having an IUD does not significantly increase the risk of acquiring sexually transmitted infections (STIs) or pelvic inflammatory disease (PID ) [26]. Therefore, being at high risk for STIs, a history of STI, or immunocompromise does not preclude the use of an IUD. However, there is an increased risk of PID when an IUD is placed in the setting of current purulent cervicitis or a known chlamydial or gonorrheal infection (U.S. MEC 4). In these cases, treatment should be initiated prior to insertion.
Significant distortion of the endometrial cavity due to fibroids, synechiae, or uterine anomalies may make IUD placement challenging, and is not recommended (U.S. MEC 4); however, determination of cavity distortion should be based on ultrasonographic findings, rather than physical exam alone. IUDs should not be initiated in women with cervical or endometrial cancer. However, the use of the LNG-IUS for women with low-grade endometrial cancer desiring fertility preservation is currently under study. Based on theoretical data, placing an IUD in the setting of gestational trophoblastic disease (GTN) with decreasing or undetectable bHCG level is generally not recommended (U.S. MEC 3). GTN with persistently elevated bHCG or malignant disease is MEC category 4.
25.2.3 Special Populations
Postpartum Women
The duration of postpartum infertility is variable and unpredictable. In non-breastfeeding women the first ovulation occurs as early as 25 days postpartum, but fertile ovulation generally does not occur until at least 42 days postpartum. In women who are exclusively breastfeeding and experience amenorrhea, infertility may extend up to 6 months postpartum, but timing of return to fertility is also unpredictable [27]. Regardless of breastfeeding status, postpartum women should not use combined hormonal contraceptives during the first 3 weeks after delivery because of concerns about increased risk for VTE (U.S. MEC 4). Limited evidence suggests that postpartum women with additional risk factors for VTE (i.e. age >35 years, obesity, cesarean delivery, smoking, etc.) generally should not use CHCs until 6 weeks after delivery (U.S. MEC 3). Due to concerns of decreased milk supply, CHCs are also not recommended in breastfeeding women until at least 4 weeks postpartum, when milk supply is generally established. All progestin-only and non-hormonal methods have been shown to be safe while breastfeeding and can be initiated immediately postpartum (U.S. MEC 1 or 2) [28].
Adolescents
Adolescents have high failure and discontinuation rates with the pill, patch, ring, DMPA, and condoms.
In addition, studies have demonstrated a twofold increased risk of contraceptive failure in young women (<21 years of age) using combined hormonal methods. Based on a large body of evidence demonstrating safety, including no evidence of increased risk of infertility, ACOG has recommended that clinicians consider LARC as first-line methods for adolescents [29]. Many young adolescents (age 14–17 years of age) seem to prefer the implant over an IUD, while older adolescents (18–20 years) favor the IUD. When consistently offered to adolescents and young women, these methods have been shown to have high rates of initiation, satisfaction, and continuation, and lead to a decrease in the risk of unintended pregnancy among adolescent women [30].
25.3 Contraceptive Methods
25.3.1 Long-Acting Reversible Contraception
Long-acting reversible contraceptive (LARC) methods include contraceptive implants and IUDs. LARC methods are highly effective (<1% failure rate), safe, and have high user continuation and satisfaction [31–33]. Effectiveness of these methods does not depend on user adherence, and thus, typical use effectiveness is similar to perfect use effectiveness.
Contraceptive Implants
Contraceptive implants consist of one or more rods containing a progestin that are inserted subdermally. Currently, the only contraceptive implant available in the USA is an etonogestrel (ENG)-releasing single-rod implant, Nexplanon®. Etonogestrel is a metabolite of desogestrel, a third generation progestin with low androgenicity. The ENG implant is FDA-approved for 3 years of use; however, emerging data indicate that it may be effective for a longer period of use. The ENG implant is the most effective contraceptive method currently available with a 0.05% annual failure rate. The implant provides contraceptive protection through suppression of ovulation and thickening of the cervical mucus. Some non-contraceptive benefits of the implant include improved acne, dysmenorrhea, and relief of pelvic pain due to endometriosis. The implant is associated with a rapid return to fertility, with resumption of ovulation within 3 weeks of removal. One study evaluating 200 parous implant users found that 96% of women using no contraception following removal of their implant conceived within 12 months [34]. The primary reason for discontinuation reported by users of the implant is irregular and unpredictable bleeding, where bleeding patterns can range from amenorrhea to frequent or prolonged bleeding. Bleeding patterns are highly variable, but the most commonly reported patterns are amenorrhea and infrequent bleeding (see ◘ Fig. 25.1). Other less commonly reported reasons for discontinuation include weight gain, emotional lability/mood changes, headaches, and breast discomfort [35].
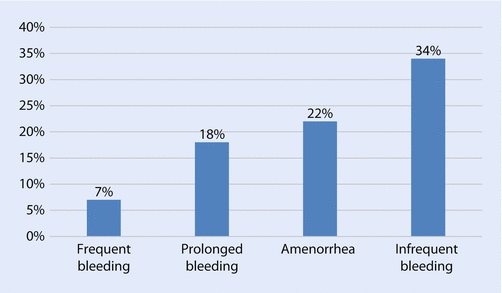

Fig. 25.1
Bleeding patterns associated with the single-rod etonogestrel -containing subdermal contraceptive implant [36]. Data source: Mansour D, Korver T, Marintcheva-Petrova M, & Fraser IS. The effects of Implanon® on menstrual bleeding patterns. Eur J Contracept Reprod Health Care, 2008; 13(Suppl 1):13–28
As stated above, the implant can be used in women who are postabortion, postpartum, and lactating. There is no evidence that the implant significantly impacts breastfeeding or newborn growth. The ENG implant is also safe and effective in special populations of women including adolescents, overweight and obese women, and women with contraindications to estrogen use.
Intrauterine Devices
Intrauterine devices (IUDs) are plastic T-shaped devices, often containing an additional active ingredient, with a string attached to one end of the device. IUDs are inserted into the uterus by a trained clinician. There are five type of IUDs currently used in the USA: 4 levonorgestrel (LNG)-containing IUDs (Mirena®, Liletta®, Kyleena® and Skyla®) and one copper (Cu)-containing IUD (ParaGard®).
25.3.2 Contraceptive Effects
Hormonal IUDs (also called LNG-IUS: levonorgestrel-releasing system) prevent pregnancy by thickening the cervical mucus, suppressing the endometrium, impairing sperm function, and partially suppressing ovulation. They have a 0.1–0.2% yearly failure rate [31]. The LNG-containing IUDs have low levels of systemic absorption of levonorgestrel. Thus, these methods do not typically produce progestin-like side effects. Both the Mirena® & Liletta® IUDs contain 52 mg of levonorgestrel, while the Kyleena® and Skyla® contain lower doses (19.5mg and 13mg, respectively) and have smaller frames. Mirena® and Kyleena® are FDA-approved for 5 years (but appear to be effective for at least 6–7 years), while the Liletta® and Skyla® are approved for 3 years. Liletta® follow-up studies are still on-going, but there is no reason to suspect it will have a shorter efficacy period than the Mirena® IUD IUD, which appears to be effective for 6-7 years. The Cu IUD primarily prevents sperm function and has a 0.5–0.8% yearly failure rate. In addition, the Cu IUD is the most effective method of emergency contraception available [37].
IUDs can be placed immediately postabortion or postpartum (ideally within 10 minutes after delivery of the placenta), and are safe for women who are breastfeeding [28]. They are also safe and well tolerated in special populations of women, including adolescents. Fertility returns promptly after removal of the hormonal or copper IUD, and in the majority of women attempting pregnancy after removal of IUD will conceive within 3–6 months after IUD removal. There are no long-term effects on fertility after long-term use of an IUD [38]. There is an approximately 5–10% rate of IUD expulsion, with higher rates reported with immediate postpartum placement.
25.3.3 Bleeding Patterns
The two most common side effects and cause for discontinuation of all IUDs are changes in menstrual bleeding pattern and pain or discomfort. Immediately after IUD insertion, many women experience cramping and irregular bleeding. This typically resolved in days to weeks, but sometimes lasts for longer periods of time. If pain symptoms persist, an evaluation to ensure correct positioning of the IUD is warranted.
LNG-IUS
Non-contraceptive benefits of the LNG-IUS include a decrease in dysmenorrhea, menstrual blood loss, and improvement in bleeding associated with adenomyosis and leiomyomas. Due to this, the LNG-IUS is increasingly being used as the primary mode of conservative management for abnormal uterine bleeding (AUB ) due to a variety of etiologies. In many patients, especially those with AUB, amenorrhea may be a beneficial side-effect. Amenorrhea rates have been shown to vary from 15 to 20% by 1 year of use. Women with light or moderate menstrual flow prior to LNG-IUS use are more likely than those with heavier bleeding to report amenorrhea [39]. However, most women report improvement in the amount of bleeding they experience. Because the bleeding experienced may be irregular and unpredictable and some women may not like being amenorrheic, change in bleeding profile can be undesirable and lead to discontinuation.
Copper IUD
Cu IUD users can experience heavier, longer, and/or more painful menses for the first few cycles after insertion. However, cramping and bleeding tends to improve over time. Women using the copper or hormonal IUD report similar long-term satisfaction and continuation rates. Both types of IUDs, copper and hormonal, are associated with a decreased risk of endometrial cancer.
25.3.4 Injectable Contraceptives
Injectable contraceptives are used worldwide and come in many formulations with periods of efficacy ranging from one to 3–4 months. Some require administration every month, others require administration every 3 months. Depot medroxyprogesterone acetate (DMPA) , a depot form of a pregnane progesterone very similar to naturally occurring progesterone, is the only injectable available in the USA. DMPA comes in both intramuscular and subcutaneous injections that are administered every 12 weeks (however, contraceptive effectiveness typically lasts 16 weeks). The primary mechanism of action is ovulation inhibition. With typical use, DMPA has a 6% yearly failure rate; however, this rate is much lower with consistent injections every 3 months. Non-contraceptive benefits of DMPA include a decrease in menstrual blood loss, dysmenorrhea, luteal phase symptoms, discomfort from endometriosis and myomas, endometrial cancer, and sickle cell crises.
Side Effects
The most common reported reason for DMPA discontinuation is change in bleeding pattern, which can range from amenorrhea to prolonged and/or irregular bleeding. Other common causes for discontinuation include weight gain and headaches. For some women, a major disadvantage to DMPA use is its association with a delayed return to fertility. Studies have shown that time to first ovulation is variable, and on average occurs 14–49 weeks from the last injection, with the median delay to conception being 9–10 months after the last injection [40].
Bone Mineral Density
Historically, concern has been raised regarding the association of DMPA use with decreased bone mineral density (BMD ). However, studies have demonstrated that DMPA users experienced a temporary and reversible loss in bone density but no increased risk of fracture [41]. As such, the benefits of preventing unintended pregnancy outweigh the theoretical risks associated with decreased BMD, and no restrictions should be placed on the use of DMPA by adolescents or on the duration of DMPA use. The only absolute contraindication to DMPA use is current breast cancer.
25.3.5 Combined Hormonal Contraceptives (CHCs)
Combined hormonal contraceptives (CHCs ) are methods containing both an estrogen and progestin. CHC formulations currently available include the combined oral contraceptive pill, transdermal patch, and contraceptive vaginal ring. Evidence indicates that the patch and the ring provide comparable safety and pharmacokinetic profiles to pills with similar hormonal formulations. Therefore, much of the evidence related to the benefits, risks, and side effects are extrapolated from pills to other methods.
Hormonal Composition
Pill formulations are based on the dosage of ethinyl estradiol, the type of progestin, and the length of the pill-free interval (see ◘ Table 25.2 for examples). Ethinyl estradiol is a synthetic estrogen formulated to impede first-pass metabolism by hepatic degeneration, thus creating a more bioavailable oral formulation. The most commonly used pills have 30–35 mcg of ethinyl estradiol, but estrogen dose can be as high as 50 mcg or as low as 10 mcg. Doses less than 30 mcg may further decrease the risk of VTE. However, doses lower than 30 mcg result in greater breakthrough bleeding and may have less effect on prevention of ovarian cyst formation and other non-contraceptive benefits of CHCs. The only pill currently available in the USA with an estrogen other than ethinyl estradiol is Natazia¸ which contains estradiol valerate and dienogest. This formulation may be superior to ethinyl estradiol preparations in the treatment of heavy menstrual bleeding, with effects similar to the hormonal IUD [42].
Table 25.2
Examples of currently available pill formulations
Name | Estrogen (EE = ethinyl estradiol) | Progestin | Cycle length (hormone/hormone-free intervals) | |
|---|---|---|---|---|
Usual dosing regimens | Lo Loestrin® | 10 mcg EE | 1 mg norethindrone | 21/7 |
Alesse® | 20 mcg EE | 0.1 mg levonorgestrel | 21/7 | |
Loestrin® | 20 mcg EE | 1 mg norethindrone | 21/7 | |
Yasmin® | 30 mcg EE | 3 mg drosperinone | 21/7 | |
Desogen® | 30 mcg EE | 0.15 mg desogestrel | 21/7 | |
Sprintec® | 35 mcg EE | 0.25 mg norgestimate | 21/7 | |
Ovral® | 50 mcg EE | 0.5 mg norgestrel | 21/7 | |
Extended dosing regimens | Lo Seasonique® | 10 mcg/20 mcg EE | 0.1 mg levonorgestrel | 84/7 |
Yaz®
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|