Systemic Lupus Erythematosus
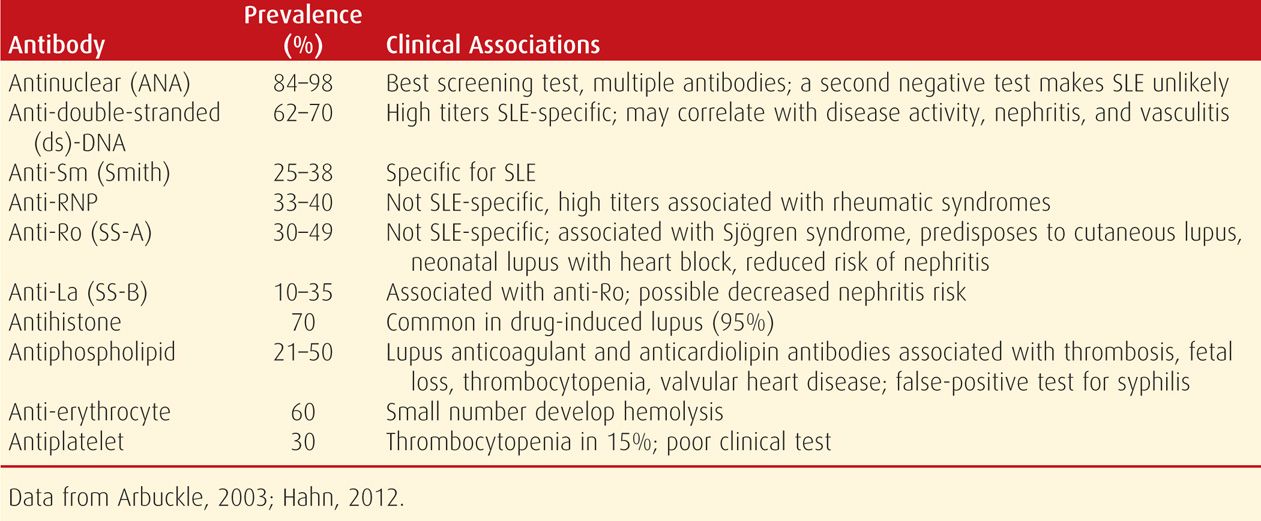
Lupus is a heterogeneous autoimmune disease with a complex pathogenesis that results in interactions between susceptibility genes and environmental factors (Hahn, 2012; Tsokos, 2011). Immune system abnormalities include overactive B lymphocytes that are responsible for autoantibody production. These result in tissue and cellular damage when autoantibodies or immune complexes are directed at one or more cellular nuclear components (Tsokos, 2011). In addition, immunosuppression is impaired, including regulatory T-cell function (Tower, 2013). Some autoantibodies produced in patients with lupus are shown in Table 59-1.
TABLE 59-1. Some Autoantibodies Produced in Patients with Systemic Lupus Erythematosus (SLE)
Almost 90 percent of lupus cases are in women, and its prevalence in those of childbearing age is approximately 1 in 500 (Lockshin, 2000). Accordingly, the disease is encountered relatively frequently during pregnancy. The 10-year survival rate is 70 to 90 percent (Hahn, 2012; Tsokos, 2011). Infection, lupus flares, end-organ failure, hypertension, stroke, and cardiovascular disease account for most deaths.
Genetic influences are implicated by a higher concordance with monozygotic compared with dizygotic twins—25 versus 2 percent, respectively. Moreover, there is a 10-percent frequency in patients with one affected family member. The relative risk of disease is increased if there is inheritance of the “autoimmunity gene” on chromosome 16 that predisposes to SLE, rheumatoid arthritis, Crohn disease, and psoriasis (Hahn, 2012). Susceptibility genes such as HLA-A1, B8, DR3, DRB1, and TET3 explain only a portion of the genetic heritability (Hom, 2008; Tsokos, 2011; Yang, 2013).
Clinical Manifestations and Diagnosis
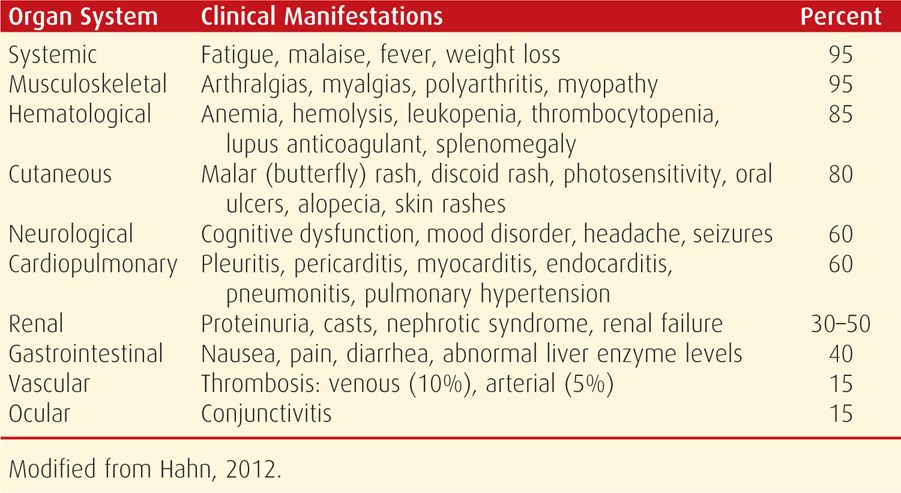
Lupus is notoriously variable in its presentation, course, and outcome (Table 59-2). Findings may be confined initially to one organ system, and others become involved later. Or, the disease may first manifest with multisystem involvement. Common findings are malaise, fever, arthritis, rash, pleuropericarditis, photosensitivity, anemia, and cognitive dysfunction. At least half of patients have renal involvement. There is evidence that lupus is associated with decline in attention, memory, and reasoning (Kozora, 2008). Although Libman-Sacks endocarditis was described with lupus, it is likely due to presence of subsequently discussed anticardiolipin antibodies (Hojnik, 1996).
TABLE 59-2. Clinical Manifestations of Systemic Lupus Erythematosus
Identification of antinuclear antibodies (ANA) is the best screening test. However, a positive result is not specific for lupus. For example, low titers are found in normal individuals, other autoimmune diseases, acute viral infections, and chronic inflammatory processes. Several drugs can also cause a positive reaction. Antibodies to double-stranded DNA (dsDNA) and to Smith (Sm) antigens are relatively specific for lupus, whereas other antibodies are not (see Table 59-1). Although hundreds of autoantibodies have been described in SLE, only a few have been shown to participate in tissue injury (Sherer, 2004; Tsokos, 2011). Microarray profiles are being developed for customized and more accurate SLE diagnoses (Lin, 2013; Yeste, 2013).
Anemia develops frequently, and there may be leukopenia and thrombocytopenia. Proteinuria and casts are found in the half of patients with glomerular lesions. Lupus nephritis can also cause renal insufficiency, which is more common if there are antiphospholipid antibodies (Moroni, 2004). Other laboratory findings include false-positive syphilis serology, prolonged partial thromboplastin time, and higher rheumatoid factor levels. Elevated serum D-dimer levels often follow a flare or infection, but unexplained persistent elevations are associated with a high risk for thrombosis (Wu, 2008).
The diagnostic criteria for SLE are listed in Table 59-3. If any four or more of these 11 criteria are present, serially or simultaneously, the diagnosis of lupus is made.
TABLE 59-3. Criteria of the American Rheumatism Association for Systemic Lupus Erythematosus (SLE)

Importantly, numerous drugs can induce a lupus-like syndrome. These include procainamide, quinidine, hydralazine, α-methyldopa, phenytoin, and phenobarbital. Drug-induced lupus is rarely associated with glomerulonephritis and usually regresses when the medication is discontinued (Rubin, 1997).
Lupus and Pregnancy
Of nearly 16.7 million pregnancies in the United States from 2000 to 2003, 13,555 were complicated by lupus—an incidence of approximately 1 in 1250 pregnancies (Clowse, 2008). During the past several decades, pregnancy outcomes in women with SLE have improved remarkably. Important factors for pregnancy outcome include whether disease is active at the beginning of pregnancy, age and parity, coexistence of other medical or obstetrical disorders, and whether antiphospholipid antibodies are detected (p. 1173). There is evidence that newly diagnosed lupus during pregnancy tends to be severe (Zhao, 2013).
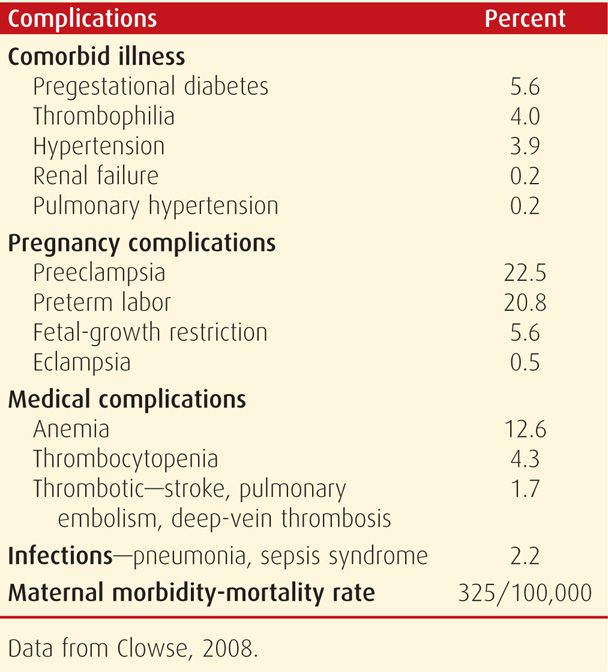
During pregnancy, lupus improves in a third of women, remains unchanged in a third, and worsens in the remaining third. Thus, in any given pregnancy, the clinical condition can worsen or flare without warning (Khamashta, 1997). Petri (1998) reported a 7-percent risk of major morbidity during pregnancy. Women who have confined cutaneous lupus do not usually have adverse outcomes (Hamed, 2013). Frequent complications in a cohort of 13,555 women with SLE during pregnancy are shown in Table 59-4. The maternal mortality and severe morbidity rate was 325 per 100,000. In a review of 13 studies with 17 maternal deaths attributable to SLE and lupus nephritis, all occurred in those with active disease (Ritchie, 2012).
TABLE 59-4. Complications in 13,555 Pregnancies in Women with Systemic Lupus Erythematosus
From the foregoing, it is certain that lupus can be life threatening for both mother and fetus. Generally speaking, pregnancy outcome is best in those women in whom: (1) lupus activity has been quiescent for at least 6 months before conception; (2) there is no lupus nephritis manifest by proteinuria or renal dysfunction; (3) there is no evidence of the antiphospholipid antibody syndrome or lupus anticoagulant; and (4) superimposed preeclampsia does not develop (Peart, 2014; Stojan, 2012).
Lupus Nephritis. Active nephritis has been associated with particularly bad pregnancy outcomes, although these have improved remarkably during the past 30 years (Moroni, 2005; Stojan, 2012). Women with renal disease have a high incidence of gestational hypertension and preeclampsia. However, if their disease remains in remission, they usually have good pregnancy outcomes (Huong, 2001; Moroni, 2002). Of the 125 pregnancies reported by Lockshin (1989), 63 percent of women with preexisting renal disease developed preeclampsia compared with only 14 percent of those without underlying renal disease. Moroni and Ponticelli (2005) reviewed results from a total of 309 pregnancies complicated by established lupus nephritis. Of these, 30 percent suffered a flare, and 40 percent of these had associated renal insufficiency. The maternal mortality rate was 1.3 percent.
In two studies describing pregnancy outcomes in women with lupus nephritis, Wagner and coworkers (2009) compared outcomes of 58 women cared for during 90 pregnancies at the Mayo Clinic. Active nephritis was associated with a significantly higher incidence of maternal complications compared with women without nephritis—57 versus 11 percent. Quiescent nephritis had a nonsignificant effect on preeclampsia rates compared with lupus patients without renal impairment. The fetal death rate with active maternal nephritis was 35 percent compared with 9 percent in those with quiescent nephritis. In another study, Imbasciati and associates (2009) described outcomes in 113 pregnancies in 81 women with known lupus nephritis. During a third of pregnancies, there was a renal flare. After excluding nine miscarriages, of the 104 remaining pregnancies, a third were delivered preterm, a third of infants weighed < 2500 g, and the perinatal mortality rate was 6 percent.
Most recommend continuation of immunosuppressive therapy for nephritis during pregnancy. It is not clear whether the dose should be increased peripartum. Although it is often stated that this is the time that activation or exacerbations are most likely to develop, the evidence is not conclusive.
Lupus versus Preeclampsia-Eclampsia. Chronic hypertension complicates up to 30 percent of pregnancies in women with SLE (Egerman, 2005). And as discussed, preeclampsia is common, and superimposed preeclampsia is encountered even more often in those with nephritis or antiphospholipid antibodies (Bertsias, 2008). It may be difficult, if not impossible to differentiate lupus nephropathy from severe preeclampsia if the kidney is the only involved organ (Petri, 2007). Central nervous system involvement with lupus may culminate in convulsions similar to those of eclampsia. Thrombocytopenia, with or without hemolysis, may further confuse the diagnosis because of its similarity to the hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome. Management is identical to that for preeclampsia-eclampsia, described in Chapter 40 (p. 749).
Management During Pregnancy
Lupus management consists primarily of monitoring maternal clinical and laboratory conditions as well as fetal well-being (Lateef, 2012). Pregnancy-induced thrombocytopenia and proteinuria resemble lupus disease activity, and the identification of a lupus flare is confounded by the increase in facial and palmar erythema of normal pregnancy (Lockshin, 2003). Some authorities have advocated a number of numerical scales to emphasize ongoing disease activity. Components are weighted for severity, both with the SLE-Pregnancy Disease Activity Index (SLEPDAI) and with the Lupus Activity Index (Buyon, 1999; Ruiz-Irastorza, 2004). We have not found these to be useful.
Lupus activity monitoring and identification of lupus flares by various laboratory techniques has been recommended. The sedimentation rate may be misleading because of pregnancy-induced hyperfibrinogenemia. Serum complement levels are also normally increased in pregnancy (Chap. 4, p. 56 and Appendix, p. 1291). And, although falling or low levels of complement components C3, C4, and CH50 are more likely to be associated with active disease, higher levels provide no assurance against disease activation. Our experiences, as well as those of Varner and colleagues (1983) and Lockshin and Druzin (1995), are that there is no correlation between clinical manifestations of disease and complement levels.
Serial hematological studies may detect changes in disease activity. Hemolysis is characterized by a positive Coombs test, anemia, reticulocytosis, and unconjugated hyperbilirubinemia. Thrombocytopenia, leukopenia, or both may develop. According to Lockshin and Druzin (1995), chronic thrombocytopenia in early pregnancy may be due to antiphospholipid antibodies. Later, thrombocytopenia may indicate preeclampsia.
Serum aminotransferase activity reflects hepatic involvement, as does a rise in serum bilirubin levels. Azathioprine therapy also may induce enzyme elevations. Urine is tested frequently to detect new-onset or worsening proteinuria. Overt proteinuria that persists is an ominous sign, even more so if accompanied by other evidence of the nephrotic syndrome or abnormal serum creatinine levels.
The fetus should be closely observed for adverse effects such as growth restriction and oligohydramnios. Many recommend screening for anti-SS-A (anti-Ro) and anti-SS-B (anti-La) antibodies, because of associated fetal complications described subsequently. As discussed in Chapter 17 (p. 335), antepartum fetal surveillance is done as outlined by the American College of Obstetricians and Gynecologists (2012a). Unless hypertension develops or there is evidence of fetal compromise or growth restriction, pregnancy is allowed to progress to term. Peripartum corticosteroids in “stress doses” are given to women who are taking these drugs or who recently have done so.
Pharmacological Treatment. There is no cure, and complete remissions are rare. Approximately a fourth of women have mild disease, which is not life threatening, but may be disabling because of pain and fatigue. Arthralgia and serositis can be managed by occasional doses of nonsteroidal antiinflammatory drugs (NSAIDs). However, chronic or large intermittent dosing is avoided due to pregnancy side effects with these drugs described in Chapter 12 (p. 247) (Briggs, 2011). Low-dose aspirin can be used throughout gestation. Severe disease is managed with corticosteroids such as prednisone, 1 to 2 mg/kg orally per day. After the disease is controlled, this dose is tapered to a daily morning dose of 10 to 15 mg. Corticosteroid therapy can result in the development of gestational diabetes.
Immunosuppressive agents such as azathioprine are beneficial in controlling active disease (Contreras, 2004; Hahn, 2012). In nonpregnant patients, these are usually reserved for lupus nephritis or disease that is corticosteroid resistant. Azathioprine has a good safety record during pregnancy (Fischer-Betz, 2013; Petri, 2007). Its recommended daily oral dose is 2 to 3 mg/kg. According to Buhimschi and Weiner (2009), cyclophosphamide is teratogenic, and although not usually recommended during pregnancy, severe disease may be treated after 12 weeks’ gestation. As discussed in Chapter 12 (p. 250), medications to be avoided include mycophenolate mofetil and methotrexate (Anderka, 2009; Briggs, 2011; Food and Drug Administration, 2008). In some situations, mycophenolate is the only treatment that achieves disease stability. In these cases, counseling regarding fetal risks is essential (Bramham, 2012).
Antimalarials help control skin disease. Although these agents cross the placenta, hydroxychloroquine has not been associated with congenital malformations. Because of the long half-life of antimalarials and because discontinuing therapy can precipitate a lupus flare, most recommend their continuation during pregnancy (Borden, 2001; Harris, 2002). Levy and associates (2001) randomly assigned 20 pregnant women to receive hydroxychloroquine or placebo and reported improved SLEPDAI scores with hydroxychloroquine.
When severe disease supervenes—usually a lupus flare—high-dose glucocorticoid therapy is given. Petri (2007) recommends pulse therapy consisting of methylprednisolone, 1000 mg given intravenously over 90 minutes daily for 3 days, then a return to maintenance doses if possible.
Perinatal Mortality and Morbidity
Adverse perinatal outcomes are increased significantly in pregnancies complicated by lupus. These include preterm delivery, fetal-growth restriction, stillbirths, and neonatal lupus syndrome (Madazli, 2014). Outcomes are worse with a lupus flare, significant proteinuria, or renal impairment, and with chronic hypertension, preeclampsia, or both (Aggarwal, 1999; Bramham, 2012; Scott, 2002; Wagner, 2009). The observations of Lee and coworkers (2009) are worrisome. In a mouse SLE model, they showed that autoantibodies directed against the N-methyl-D-aspartate neuroreceptor caused fetal neurotoxicity. This suggests an underlying etiology for the CNS manifestations and learning disorders in children of affected mothers. Ongoing work on anti-dsDNA antibodies has identified peptides that can protect target organs from antibody-mediated damage (Diamond, 2011).
The reasons at least partially responsible for adverse fetal consequences include decidual vasculopathy with placental infarction and decreased perfusion (Hanly, 1988; Lubbe, 1984). Placental pathology is discussed in more detail in Chapter 6 (p. 119).
Neonatal Lupus Syndrome. This unusual constellation is characterized by newborn skin lesions—lupus dermatitis; a variable number of hematological and systemic derangements; and occasionally congenital heart block (Boh, 2004; Lee, 2009). Although usually associated with anti-SS-A and -SS-B antibodies, McGeachy and Lam (2009) described an affected infant in whom only anti-ribonucleoprotein (RNP) antibodies were found. Thrombocytopenia and hepatic involvement are seen in 5 to 10 percent of affected infants. One report suggests that neonatal lupus may appear up to 4 weeks after birth (Stirnemann, 2002). Lockshin and colleagues (1988) prospectively followed 91 infants born to women with lupus. Eight were possibly affected—four had definite neonatal lupus and four had possible disease. Clinical manifestations, which include cutaneous lupus, thrombocytopenia, and autoimmune hemolysis, are transient and clear within a few months (Lee, 1984). This may not be so for congenital heart block. In subsequent offspring, the recurrence risk for neonatal lupus is up to 25 percent (Julkunen, 1993).
Congenital Heart Block. Fetal and neonatal heart block results from diffuse myocarditis and fibrosis in the region between the atrioventricular (AV) node and bundle of His. Buyon and associates (1993) reported that congenital heart block developed almost exclusively in fetuses of women with antibodies to the SS-A or SS-B antigens. These antibodies may also cause otherwise unexplained stillbirths (Ottaviani, 2004). Even in the presence of such antibodies, however, the incidence of myocarditis is only 2 to 3 percent but increases to 20 percent with a prior affected child (Bramham, 2012; Lockshin, 1988). Fetal cardiac monitoring should be performed between 18 and 26 weeks’ gestation in pregnancies with either of these antibodies. The cardiac lesion is permanent, and a pacemaker is generally necessary. Long-term prognosis is poor. Of 325 infants with cardiac neonatal lupus, nearly 20 percent died, and of these, a third were stillborn (Izmirly, 2011).
Maternal administration of corticosteroids, plasma exchange, or intravenous immunoglobulin have not been found to reduce the risk of congenital heart block. Corticosteroid therapy to treat fetal heart block has not been subjected to randomized trials. There is some evidence that early treatment may mitigate fetal myocarditis. Shinohara and coworkers (1999) reported no heart block in 26 neonates whose mothers received corticosteroids before 16 weeks’ gestation as part of SLE maintenance therapy. By contrast, 15 of 61 neonates with heart block were born to women in whom corticosteroid therapy was begun after 16 weeks for SLE exacerbation. Rein and colleagues (2009) followed 70 fetuses prospectively of mothers positive for anti-SS-A or -SS-B antibodies with serial fetal kinetocardiography (FKCG) to measure AV conduction time. In six fetuses, first-degree heart block developed at 21 to 34 weeks, and maternal dexamethasone treatment was associated with normalization of AV conduction in all within 3 to 14 days. There were no recurrences, and the infants all were well at a median follow-up of 4 years. However, as noted in Chapter 16 (p. 322), corticosteroids may be less effective for higher degrees of heart block. Moreover, potential benefit should be weighed against the risks of chronic corticosteroid treatment, including impaired fetal growth (Friedman, 2009).
Long-Term Prognosis and Contraception
In general, women with lupus and chronic vascular or renal disease may limit family size because of morbidity associated with the disease as well as increased adverse perinatal outcomes. Two large multicenter clinical trials have shown that combination oral contraceptives (COCs) did not increase the incidence of lupus flares (Petri, 2005; Sánchez-Guerrero, 2005). Still, the American College of Obstetricians and Gynecologists (2013) recommends that COC use be avoided in women who have nephritis, antiphospholipid antibodies, or vascular disease. Progestin-only implants and injections provide effective contraception with no known effects on lupus flares (Chabbert-Buffet, 2010). Concerns that intrauterine device (IUD) use and immunosuppressive therapy lead to increased infection rates in these patients are not evidenced-based. Tubal sterilization may be advantageous and is performed with greatest safety postpartum or whenever the disease is quiescent. These options are discussed in further detail in Chapters 38 and 39.
 Antiphospholipid Antibody Syndrome
Antiphospholipid Antibody Syndrome
Phospholipids are the main lipid constituents of cell and organelle membranes. There are proteins in plasma that associate noncovalently with these phospholipids. Antiphospholipid antibodies are directed against these phospholipids or phospholipid-binding proteins (Erkan, 2011; Giannakopoulos, 2013; Tsokos, 2011). This antibody group may be of IgG, IgM, and IgA classes, alone or in combination. Antiphospholipid antibodies are most common with lupus and other connective-tissue disorders, although a small proportion of otherwise normal women and men are found to have these antibodies in various forms.
The stimulus for autoantibody production is unclear, but it possibly is due to a preceding infection. The pathophysiology encountered in the antiphospholipid antibody syndrome—variably referred to as APAS or APS—is mediated by one or more of the following: (1) activation of various procoagulants, (2) inactivation of natural anticoagulants, (3) complement activation, and (4) inhibition of syncytiotrophoblast differentiation (Moutsopoulos, 2012; Tsokos, 2011). Clinically, these result in arterial or venous thromboses or pregnancy morbidity, and virtually every organ system may be involved as shown in Table 59-5.
TABLE 59-5. Some Clinical Features of Antiphospholipid Antibody Syndrome
Venous thrombosis—thromboembolism, thrombophlebitis, livedo reticularis
Arterial thrombosis—stroke, transient ischemic attack, Libman-Sacks cardiac vegetations, myocardial ischemia, distal extremity and visceral thrombosis and gangrene
Hematological—thrombocytopenia, autoimmune hemolytic anemia
Other—neurological manifestations, migraine headaches, epilepsy; renal artery, vein, or glomerular thrombosis; arthritis and arthralgia
Pregnancy—preeclampsia syndrome, recurrent miscarriage, fetal death
Central nervous system involvement is one of the most prominent clinical manifestations. In addition to cerebrovascular arterial and venous thrombotic events, there may be psychiatric features and even multiple sclerosis (Binder, 2010; Sanna, 2003). Renovascular involvement may lead to renal failure that may be difficult to differentiate from lupus nephritis (D’Cruz, 2009). Peripheral and visceral thromboses are also a feature. For example, Ahmed and associates (2009) reported a postpartum woman who developed spontaneous cecal perforation associated with a mesenteric vessel infarction. As discussed in Chapter 18 (p. 359), antiphospholipid antibodies have been associated with excessive pregnancy loss (Branch, 2010). These and other detrimental effects on pregnancy outcomes are discussed subsequently.
A small proportion of these patients develop the catastrophic antiphospholipid antibody syndrome—CAPS. This is defined as a rapidly progressive thromboembolic disorder simultaneously involving three or more organ systems or tissues (Moutsopoulos, 2012).
Specific Antiphospholipid Antibodies
Several autoantibodies have been described that are directed against a specific phospholipid or phospholipid-binding protein. First, β2-glycoprotein I—also known as apolipoprotein H—is a phospholipid-binding plasma protein that inhibits prothrombinase activity within platelets and inhibits ADP-induced platelet aggregation (Giannakopoulos, 2013; Shi, 1993). Thus, its normal action is to inhibit procoagulant binding and thereby prevent coagulation cascade activation. Logically, antibodies directed against β2-glycoprotein I would inhibit its anticoagulant activity and promote thrombosis. This is important from an obstetrical viewpoint because β2-glycoprotein I is expressed in high concentrations on the syncytiotrophoblastic surface. Complement activation may contribute to it pathogenesis (Avalos, 2009; Tsokos, 2011). Teleologically, this seems appropriate because the decidua intuitively should be a critical area to prevent coagulation that might result in intervillous space thrombosis. Another possibility is that β2-glycoprotein I may be involved in implantation, and it may result in pregnancy loss via an inflammatory mechanism (Iwasawa, 2012; Meroni, 2011).
Second, lupus anticoagulant (LAC) is a heterogeneous group of antibodies directed also against phospholipid-binding proteins. LAC induces prolongation in vitro of the prothrombin, partial thromboplastin, and Russell viper venom times (Feinstein, 1972). Thus, paradoxically, this so-called anticoagulant is actually powerfully thrombotic in vivo.
Last, there are anticardiolipin antibodies (ACAs). These are directed against one of the many phospholipid cardiolipins found in mitochondrial membranes and platelets.
Antibodies against Natural Anticoagulants
Antiphospholipid antibodies have also been described that are directed against the natural anticoagulant proteins C and S (Robertson, 2006). Another is directed against the anticoagulant protein annexin V, which is expressed in high concentrations by the syncytiotrophoblast (Chamley, 1999; Giannakopoulos, 2013). That said, criteria to diagnosis antiphospholipid-antibody syndrome require testing for elevated levels of the following antiphospholipid antibodies: LAC, ACA, and anti-β2 glycoprotein I. Testing for other antibodies is not recommended (American College of Obstetricians and Gynecologists, 2012b).
Antiphospholipid Antibody Syndrome Diagnosis
Clinical classification criteria shown in Table 59-5 provide indications for testing. By international consensus, the syndrome is diagnosed based on laboratory and clinical criteria (Miyakis, 2006). First, one of two clinical criteria—which are vascular thrombosis or certain pregnancy morbidity—must be present. In addition, at least one laboratory criterion that includes LAC activity or medium- to high-positive levels of specific IgG- or IgM-ACAs must be confirmed on two occasions 12 weeks apart.
Tests for LAC are nonspecific coagulation tests. The partial thromboplastin time is generally prolonged because the anticoagulant interferes with conversion of prothrombin to thrombin in vitro. Tests considered more specific are the dilute Russell viper venom test and the platelet neutralization procedure. There is currently disagreement as to which of these two is best for screening. If either is positive, then identification of LAC is confirmed.
Branch and Khamashta (2003) recommend conservative interpretation of results based on repeated tests from a reliable laboratory that are consistent with each clinical case. Only approximately 20 percent of patients with APS have a positive LAC reaction alone. Thus, anticardiolipin enzyme-linked immunosorbent assay (ELISA) testing should also be performed. Many laboratories have a premade panel to use for APS testing.
Efforts have been made to standardize ACA assays by using ELISA. Values are reported in units and expressed as negative, low-positive, medium-positive, or high-positive (Harris, 1987a,b). Despite this, these assays remain without international standardization (Adams, 2013; Branch, 2003; Capuano, 2007). Interlaboratory variation can be large, and agreement between commercial kits is poor.
Pregnancy and Antiphospholipid Antibodies
Nonspecific low levels of antiphospholipid antibodies are identified in approximately 5 percent of normal adults (Branch, 2010). When Lockwood and coworkers (1989) first studied 737 normal pregnant women, they reported that 0.3 percent had lupus anticoagulant and 2.2 percent had elevated concentrations of either IgM or IgG anticardiolipin antibodies. Later studies confirmed this, and taken together, they totaled almost 4000 normal pregnancies with an average prevalence for APAs of 4.7 percent. This is the same as for normal nonpregnant individuals (Harris, 1991; Pattison, 1993; Yasuda, 1995).
In women with high levels of ACAs, and especially when lupus anticoagulant is identified, there are increased risks for decidual vasculopathy, placental infarction, fetal-growth restriction, early-onset preeclampsia, and recurrent fetal death. Some of these women, like those with lupus, also have a high incidence of venous and arterial thromboses, cerebral thrombosis, hemolytic anemia, thrombocytopenia, and pulmonary hypertension (American College of Obstetricians and Gynecologists, 2012b; Clowse, 2008). In 191 LAC-negative women with antiphospholipid-antibody syndrome, women with antibodies to cardiolipin and β2-glycoprotein I had significantly higher miscarriage rates than if either one alone was positive (Liu, 2013).
Pregnancy Pathophysiology. It is not precisely known how these antibodies cause damage, but it is likely that their actions are multifactorial (Tsokos, 2011). Platelets may be damaged directly by antiphospholipid antibody or indirectly by binding β2-glycoprotein I, which causes platelets to be susceptible to aggregation (Chamley, 1999; Giannakopoulos, 2013). Rand and colleagues (1997a,b, 1998) propose that phospholipid-containing endothelial cell or syncytiotrophoblast membranes may be damaged directly by the antiphospholipid antibody or indirectly by antibody binding to either β2-glycoprotein I or annexin V. This prevents the cell membranes from protecting the syncytiotrophoblast and endothelium. It results in exposure of basement membrane to which damaged platelets can adhere and form a thrombus (Lubbe, 1984).
Pierro and associates (1999) reported that antiphospholipid antibodies decreased decidual production of the vasodilating prostaglandin E2. Decreased protein C or S activity and increased prothrombin activation may also be contributory (Ogunyemi, 2002; Zangari, 1997). Amengual and coworkers (2003) presented evidence that thrombosis with APS is due to activation of the tissue factor pathway. Finally, uncontrolled placental complement activation by antiphospholipid antibodies may play a role in fetal loss and growth restriction (Holers, 2002).
Complications from APS cannot be completely explained by thrombosis alone. Animal models suggest that these are due to inflammation rather than thrombosis (Cohen, 2011). Some investigators theorize that APS-associated clotting is triggered by a “second hit” from innate inflammatory immune responses and recommend antiinflammatory agents (Meroni, 2011).
Adverse Pregnancy Outcomes. Antiphospholipid antibodies are associated with increased rates of fetal wastage (Chap. 18, p. 359). In most early reports, however, women were included because they had had repeated adverse outcomes. Both are common—recall that the incidence of antiphospholipid antibodies in the general obstetrical population is about 5 percent and early pregnancy loss approximates 20 percent. Accordingly, data currently are too limited to allow precise conclusions to be drawn concerning the impact of these antibodies on adverse pregnancy outcomes. Fetal deaths, however, are more characteristic with APS than are first-trimester miscarriages (Oshiro, 1996; Roque, 2001). It has also been shown that women with higher antibody titers have worse outcomes compared with those with low titers (Nodler, 2009; Simchen, 2009).
Looking at the issue another way, the frequency of antiphospholipid antibodies may be increased with associated adverse obstetrical outcomes. Polzin and colleagues (1991) identified antiphospholipid antibodies in a fourth of 37 women with growth-restricted fetuses, however, none had evidence for lupus anticoagulant. Approximately a third of women with APS will develop preeclampsia during pregnancy (Clark, 2007b). Moodley and associates (1995) found antiphospholipid antibodies in 11 percent of 34 women with severe preeclampsia before 30 weeks.
When otherwise unexplained fetal deaths are examined, the data are mixed. Haddow and coworkers (1991) measured ACAs in 309 pregnancies with fetal death and found no differences compared with those in 618 normal pregnancies. In women with a history of recurrent pregnancy loss, those with antiphospholipid antibodies had a higher rate of preterm delivery (Clark, 2007a). In a case-control study of 582 stillbirths and 1547 live births, Silver and colleagues (2013) found a three- to fivefold increased risk for stillbirth in women with elevated anticardiolipin and anti-β2-glycoprotein I levels. Due to the risk of fetal-growth abnormalities and stillbirth, serial sonographic assessment of fetal growth and antepartum testing in the third trimester are recommended by the American College of Obstetricians and Gynecologists (2012b).
Treatment in Pregnancy. Because of the heterogeneity of studies, current treatment recommendations for women with antiphospholipid antibodies can be confusing (Branch, 2003; Robertson, 2006). As discussed, antiphospholipid antibodies that bind to immunoglobulins G, M, and A are semiquantified, and GPL, MPL, and APL binding units are expressed as negative, low-positive, medium-positive, or high-positive (American College of Obstetricians and Gynecologists, 2012b). Of the three, higher titers for GPL and MPL anticardiolipin antibodies are clinically important, whereas low-positive titers are of questionable clinical significance. And any titer of APL antibodies has no known relevance at this time.
As discussed in Chapter 52 (p. 1033), women with prior thromboembolic events who have antiphospholipid antibodies are at risk for recurrence in subsequent pregnancies. For these women, prophylactic anticoagulation with heparin throughout pregnancy and for 6 weeks postpartum with either heparin or warfarin is recommended (American College of Obstetricians and Gynecologists, 2012b). For those without history of thromboembolic events, recommendations for management from the American College of Obstetricians and Gynecologists (2012b) and the American College of Chest Physicians (Bates, 2012) are varied and listed in Table 52-8 (p. 1046). Some acceptable schemes include close antepartum observation with or without prophylactic or intermediate-dose heparin, and some form of postpartum anticoagulation for 4 to 6 weeks.
Recent trials have caused the need for heparin to be questioned in women with antibodies but no history of thrombosis (Branch, 2010). Although this is less clear, some recommend that women be treated if they have medium- or high-positive ACA titers or LAC activity and a previous second- or third-trimester fetal death not attributable to other causes (Dizon-Townson, 1998; Lockshin, 1995). Some report that women with recurrent early pregnancy loss and medium- or high-positive titers of antibodies may benefit from therapy (Robertson, 2006). Some individual agents are now considered for pregnant women with antiphospholipid antibody syndrome who have no prior thromboembolic event. These agents are described in the following paragraphs.
Aspirin, given in doses of 60 to 80 mg orally daily, blocks the conversion of arachidonic acid to thromboxane A2 while sparing prostacyclin production. This reduces synthesis of thromboxane A2, which usually causes platelet aggregation and vasoconstriction, and simultaneously spares prostacyclin, which normally has the opposite effect. There appear to be no major side effects from low-dose aspirin other than a slight risk of small-vessel bleeding during surgical procedures. Low-dose aspirin does not appear to reduce adverse pregnancy outcomes in antiphospholipid antibody-positive women without the complete syndrome (Del Ross, 2013).
Unfractionated heparin is given subcutaneously in dosages of 5000 to 10,000 units every 12 hours. Some prefer low-molecular-weight heparin, such as 40 mg enoxaparin once daily, because of its ease of administration and smaller risk of osteoporosis and heparin-induced thrombocytopenia (Kwak-Kim, 2013). With therapeutic dosing, some recommend measurement of heparin levels because clotting tests may be altered by lupus anticoagulant (Cowchock, 1998). The rationale for heparin therapy is to prevent venous and arterial thrombotic episodes. Heparin therapy also prevents thrombosis in the microcirculation, including the decidual-trophoblastic interface (Toglia, 1996). As discussed, heparin binds to β2-glycoprotein I, which coats the syncytiotrophoblast. This prevents binding of anticardiolipin and anti-β2-glycoprotein I antibodies to their surfaces, which likely prevents cellular damage (Chamley, 1999; Tsokos, 2011). Heparin also binds to antiphospholipid antibodies in vitro and likely in vivo. It is problematic that heparin therapy is associated with a number of complications that include bleeding, thrombocytopenia, osteopenia, and osteoporosis. A detailed description of the use of various heparins and their doses and adverse effects is found in Chapter 52 (p. 1037).
Glucocorticosteroids generally should not be used with primary APS—that is, without an associated connective-tissue disorder. For women with lupus or those being treated for APS who develop lupus, corticosteroid therapy is indicated (Carbone, 1999). In such cases of secondary APS seen with lupus, the dose of prednisone should be maintained at the lowest effective level to prevent flares.
Immunoglobulin therapy is controversial and has usually been reserved for women with either overt disease or heparin-induced thrombocytopenia, or both (Alijotas-Reig, 2013). It is used when other first-line therapies have failed, especially in the setting of preeclampsia and fetal-growth restriction (Cowchock, 1996, 1998; Heilmann, 2003; Silver, 1997). Intravenous immunoglobulin (IVIG) is administered in doses of 0.4 g/kg daily for 5 days—total dose of 2 g/kg. This is repeated monthly, or it is given as a single dose of 1 g/kg each month. Treatment is expensive, and at $75 per gram, one course for a 70-kg woman costs more than $10,000. A Cochrane review of seven trials found no improvement in the livebirth rate for immunotherapy given to women with recurrent pregnancy loss (Porter, 2006). Xiao and associates (2013) studied prednisone and aspirin in 87 patients compared with prednisone, aspirin, low-molecular-weight heparin, and IVIG in 42 women with antiphospholipid antibody syndrome. They reported livebirth rates of 84 and 98 percent and obstetrical morbidity rates of 23 and 7 percent, respectively. We are of the view that randomized trials are needed before there is widespread application of this expensive and cumbersome therapy.
Immunosuppression has not been well evaluated, but azathioprine and cyclosporine do not appear to improve standard therapies (Silver, 1997). As mentioned with lupus on page 1172, methotrexate, cyclophosphamide, and mycophenolate mofetil are contraindicated because of teratogenic potential (Briggs, 2011; Buhimschi, 2009). Hydroxychloroquine therapy may be beneficial with APS (Albert, 2014).
Aspirin plus heparin is the most efficacious regimen (de Jesus, 2014). Low-dose unfractionated heparin—7500 to 10,000 units is used subcutaneously twice daily. Concurrently, low-dose aspirin—60 to 80 mg orally once daily—is given. Although typically given in addition to heparin in women with a prior thromboembolic event, the benefit of aspirin is unclear (American College of Obstetricians and Gynecologists, 2012b; Bouvier, 2014).
Treatment Efficacy. Fetal loss is common in women with antiphospholipid antibodies if untreated (Branch, 1992; Rai, 1995). Even with treatment, recurrent fetal loss rates remain at 20 to 30 percent (Branch, 2003; Empson, 2005; Ernest, 2011). Importantly, some women with lupus and antiphospholipid antibodies have normal pregnancy outcomes without treatment. Again, it is emphasized that women with lupus anticoagulant and prior bad pregnancy outcomes have had liveborns without treatment (Trudinger, 1988).
In a manner similar to neonatal lupus syndrome (p. 1172), up to 30 percent of neonates demonstrate passively acquired antiphospholipid antibodies, and thus there is concern for their adverse effects. For example, Tincani and coworkers (2009) found increased learning disabilities in these children. Simchen and colleagues (2009) reported a fourfold increased risk for perinatal strokes. Of 141 infants followed in a European registry, the rate of preterm birth was 16 percent; low birthweight, 17 percent; and behavioral abnormalities in 4 percent of the children. There were no cases of neonatal thrombosis (Motta, 2010).
 Rheumatoid Arthritis
Rheumatoid Arthritis
This is a chronic multisystem disease of unknown cause with an immunologically mediated pathogenesis. Infiltrating T cells secrete cytokines that mediate inflammation and systemic symptoms. Its worldwide prevalence is 0.5 to 1 percent, women are affected three times more often than men, and peak onset is from 25 to 55 years (Shah, 2012). The cardinal feature is inflammatory synovitis that usually involves the peripheral joints. The disease has a propensity for cartilage destruction, bony erosions, and joint deformities. Criteria for classification are shown in Table 59-6, and a score of 6 or greater fulfills the requirements for definitive diagnosis.
TABLE 59-6. Criteria for Classification of Rheumatoid Arthritis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


