CHAPTER 10 Congenital Diaphragmatic Hernia and Eventration of the Diaphragm
Congenital Diaphragmatic Hernia
Step 1: Surgical Anatomy
♦ Congenital diaphragmatic hernia (CDH) typically refers to a defect in the posterolateral diaphragm, known as the foramen of Bochdalek. The abdominal viscera translocate through this defect into the chest during fetal development.
♦ The diaphragm is a musculotendinous dome-shaped construction that separates the thoracic and peritoneal cavities. The central tendon is a fibrous structure that accounts for about one third of the total surface area of the diaphragm and is the insertion point for the crural fibers that pass around the aorta and the esophagus and continue to the ligament of Treitz.
♦ The diaphragm develops during weeks 4 through 8 of gestation from several fused embryonic components, including the septum transversum, pleuroperitoneal membranes or folds, and the mesentery of the esophagus. The septum transversum forms the central tendon. Closure of the pleuroperitoneal membranes completes the formation of the fetal diaphragm. It is hypothesized that the posterolateral defect in the diaphragm is a result of incomplete or absent fusion of these membranes. As a consequence, the abdominal viscera migrate into the chest during the first trimester.
♦ The ipsilateral lung is affected more than the contralateral lung, but both are affected. The microstructural abnormality features compromised pulmonary arterial divisions, muscular hypertrophy of the intra-acinar arterioles, decreased bronchiolar units, and consequently a decreased surface available for gas exchange. Affected infants are born with both pulmonary hypoplasia and pulmonary hypertension. The pulmonary hypoplasia can be severe enough to preclude survival without fetal circulation, but successful management of the pulmonary hypertension can enable to infant to thrive.
Step 2: Preoperative Considerations
Diagnosis
♦ The diagnosis is typically made antenatally. The infant is usually full term and manifests respiratory distress immediately.
♦ Prenatal ultrasound demonstrates a fetus with bowel, liver, or spleen in the chest and shifting of the mediastinum.
♦ At birth the diagnosis is confirmed by chest radiograph showing loops of bowel in the thoracic cavity with contralateral mediastinal shift.
Resuscitation
♦ Prompt intubation and nasogastric decompression should be the first steps in the resuscitation process.
♦ Ventilator strategies depend on the clinical situation.
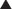 Patients should always be allowed to breathe spontaneously, and paralytic agents should be avoided to minimize barotrauma.
Patients should always be allowed to breathe spontaneously, and paralytic agents should be avoided to minimize barotrauma.
 Patients should always be allowed to breathe spontaneously, and paralytic agents should be avoided to minimize barotrauma.
Patients should always be allowed to breathe spontaneously, and paralytic agents should be avoided to minimize barotrauma.♦ Standard monitoring should be immediately instituted with the addition of preductal and postductal arterial oxygen saturation (Sao2).
 Most of these infants have significant pulmonary hypertension leading to right-to-left shunting at the patent ductus arteriosus, and therefore blood gas monitoring via the umbilical artery (postductal) is often misleading.
Most of these infants have significant pulmonary hypertension leading to right-to-left shunting at the patent ductus arteriosus, and therefore blood gas monitoring via the umbilical artery (postductal) is often misleading.
 Most of these infants have significant pulmonary hypertension leading to right-to-left shunting at the patent ductus arteriosus, and therefore blood gas monitoring via the umbilical artery (postductal) is often misleading.
Most of these infants have significant pulmonary hypertension leading to right-to-left shunting at the patent ductus arteriosus, and therefore blood gas monitoring via the umbilical artery (postductal) is often misleading.♦ Arterial and venous accesses are required to assist in the resuscitation of the infant in respiratory distress.
♦ An echocardiogram is obtained with special attention to signs of pulmonary hypertension. Pharmacologic supports that either dilate the pulmonary vasculature or assist in improving right-sided heart function are useful. Agents such as nitric oxide, iloprost, epoprostenol, sildenafil, dobutamine, and milrinone have all been used to help improve the pulmonary hypertension or its effects.
♦ Extracorporeal membrane oxygenation (ECMO, discussed in Chapter 2) is an adjunct in the preoperative infant who has already demonstrated evidence of adequate lung function but who then deteriorates secondary to pulmonary hypertension. The likelihood that this infant would tolerate surgery is low, and therefore the added cardiopulmonary support will allow the infant to recover from the acute pulmonary hypertensive crisis. Repair is typically performed when ECMO parameters are near the time of decannulation. The infant is usually weaned off ECMO support within 1 to 2 days after closure of the diaphragmatic defect.
Step 3: Operative Steps
Anesthesia
♦ An orotracheal or nasotracheal tube is used to secure the airway. A nasotracheal tube has the theoretical advantage of lower incidence of airway complications, but an orotracheal tube is easier to place and more commonly used.
♦ A pressure-cycled infant ventilator is preferred over the conventional anesthesia circuit to provide mechanical ventilation to the infant.
♦ Muscle paralysis and narcotics are administered and complemented with intravenous anesthetic agents on an as-needed basis.
Positioning
♦ The operative room should be warmed, and focus should include keeping the patient warm at all times. Plastic drapes are used to cover the child outside the operating field.
Incision
♦ The incision is made approximately one fingerbreadth below the costal margin. While incising the anterior abdominal wall musculature and fascia, be careful to leave enough fascia and muscle on the cephalad aspect of the wound to provide for an adequate closure (Fig. 10-1).
♦ Electrocautery is used to ensure hemostasis because of the potential need for ECMO and anticoagulation.
Hernia Reduction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree