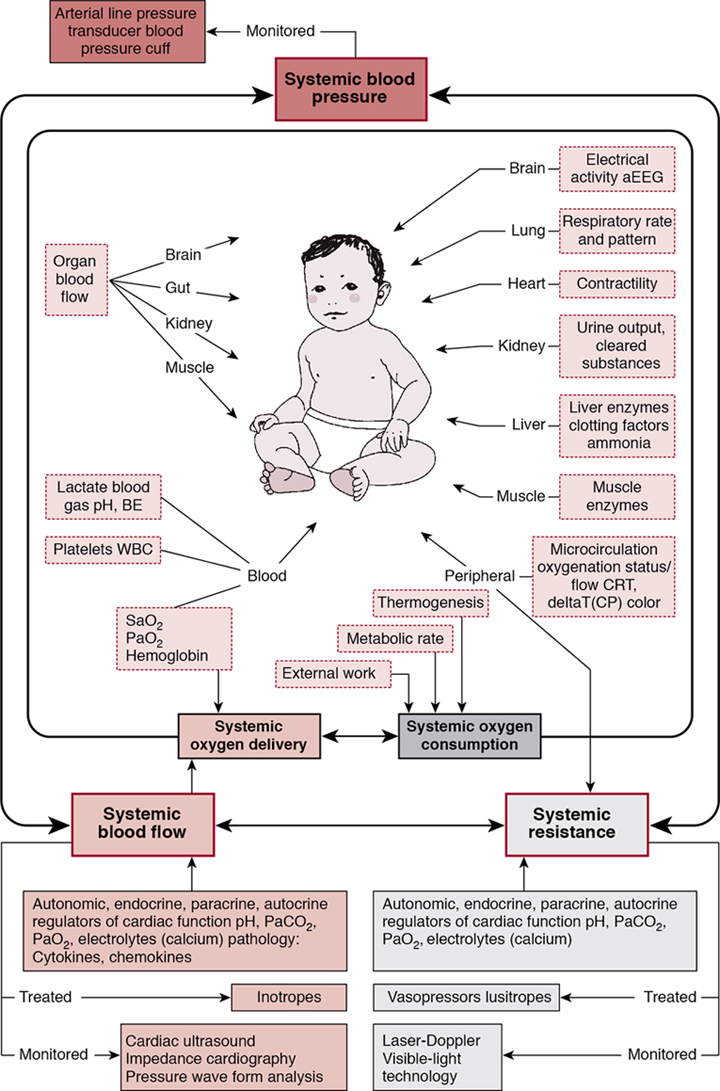
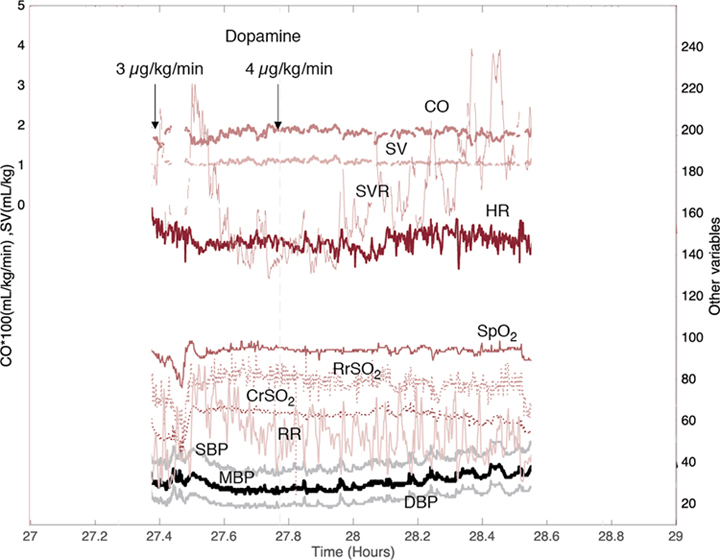
Willem-Pieter de Boode, Shahab Noori, David van Laere, Eugene Dempsey, Istvan Seri Key points Recent advances in biomedical research and technology have allowed clinicians to obtain more clinically relevant physiologic, biochemical, and genetic information that could be useful in the diagnosis and management of various conditions. Neonatology has become one of the rapidly evolving subspecialties at the frontier of this progress. However, the field of neonatal hemodynamics, while being extensively investigated in basic/animal laboratory and clinical research settings, remains inadequately understood. Accordingly, we continue to have difficulties in establishing reliable criteria for the diagnosis of the most common deviations from physiology, such as neonatal hypotension, especially during the period of immediate postnatal transition. This, in turn, leads to a paucity of established, evidence-based guidelines on when and how to intervene in a neonate presenting with these conditions.1 Thus we must recognize the significant limitations of our current understanding of a number of clinically relevant aspects of neonatal cardiovascular physiology and pathophysiology and acknowledge the existing vast differences in opinions on diagnostic criteria and treatment approaches in neonatal intensive care and neonatal cardiovascular pathophysiology in particular. The next logical step in identifying individual patients with early signs of hemodynamic compromise is to develop and implement comprehensive objective hemodynamic monitoring systems that enable continuous and real-time monitoring and acquisition of multiple hemodynamic parameters of systemic and regional blood flow and oxygen demand-delivery coupling. The information obtained can then be used to design and execute clinical trials in subpopulations of neonates exhibiting common hemodynamic features and targeted by a given intervention. This approach will enable timely identification of the individual patient in the future in whom a trial-tested, individualized management plan can be utilized and the response to the pathophysiology- and evidence-based interventions monitored. Multiple studies have shown an association between severe cardiovascular compromise and increased morbidity and mortality in affected patients.2–6 Although there is some evidence for improved outcome in hypotensive preterm infants responding to vasopressor-inotropes with increases in blood pressure and cerebral blood flow,7,8 essentially none of the suggested interventions or medications used (dopamine, epinephrine, dobutamine, milrinone or vasopressin) has been properly studied to determine the actual impact of the treatment on clinically relevant medium- and long-term outcomes.9–13 The failure to identify effective interventions for the treatment of neonatal hemodynamic compromise stems from several unresolved challenges. The cardiovascular system of the newborn undergoes rapid changes during transition to extrauterine life, and these changes are greatly affected by multiple intrinsic and extrinsic factors. Such factors include, but are not limited to, individual variations in the degree of immaturity based on gestational and postnatal age, coexisting comorbidities including the need for positive pressure ventilation, the complex interactions between systemic and regional blood flow, and underlying genetic heterogeneity. Another fundamental challenge is the lack of pathophysiology- and evidence-based definition of neonatal hypotension (see Chapters 1 and 3). Measurements of blood pressure with or without the use of indirect clinical indicators of perfusion remain the major criterion in the assessment of the hemodynamic status and the need for interventions.13–15 Normative blood pressure values in preterm and term infants have been reported in population-based studies, and mean arterial blood pressure increases with increasing gestational and postnatal age (Chapter 3).16,17 However, blood pressure within the normal range for a given gestational and postnatal age does not necessarily reflect normal organ blood flow. And, similarly, abnormally low blood pressure values do not automatically translate into compromised organ blood flow (see Chapters 1 and 3). So, for patients of the same gestational age and degree of maturity, the same blood pressure values can be associated with either adequate or compromised systemic and organ perfusion. More so, even for the same patient under different conditions and points in time, the same blood pressure values may represent adequate or compromised systemic and/or organ perfusion. The reason for such limitations of using the blood pressure alone as an indicator of hemodynamic compromise lies in the fact that blood pressure is determined by the interaction between systemic blood flow (represented by effective cardiac output) and systemic vascular resistance. Thus the same values of blood pressure, the dependent variable, can result from different combinations of the other two, independent, variables. In the early, compensated phase of shock, blood pressure remains within the normal range while non-vital organ perfusion has, by definition, decreased. As many pathophysiologic mechanisms may lead to inadequate organ blood flow, whether they affect effective cardiac output, systemic vascular resistance, or both, failure to recognize these changes potentially leads to delay in initiation of treatment, exhaustion of limited compensatory mechanisms, and a resultant progression to the uncompensated phase of shock, with obvious signs of decreased organ perfusion and oxygen delivery. On the other hand, unnecessary treatment might also be started if the condition is thought to have reached the treatment threshold when, in reality, systemic and/or regional blood flow is maintained. In addition, identification of the primary pathophysiologic mechanism that could prompt appropriately targeted intervention becomes more challenging when reliable information on the status of the macro-circulation and/or tissue oxygen delivery is not readily available. Other conventional hemodynamic parameters (heart rate and arterial oxygen saturation), even if continuously monitored along with blood pressure, as well as capillary refill time, urine output, and serum lactate levels, have significant limitations for timely and accurate assessment of both the cardiovascular status and the response to interventions aimed to treat the hemodynamic compromise. Therefore inclusion of targeted assessment of systemic blood flow and regional organ perfusion becomes paramount to overcome these limitations and identify at-risk patients in a timely manner and intervene appropriately. The essential component in bedside assessment of systemic perfusion is measurement of cardiac output (CO). Several diagnostic modalities are available for such measurements, with functional cardiac MRI (fcMRI) being now considered a “gold standard”. However, several factors, such as the need for expensive equipment and highly trained personnel and sedation and transportation of the patient to the MRI suite, as well as its non-continuous nature limit the use of fcMRI for routine bedside assessment.18 Accordingly, fcMRI remains mostly utilized in research settings (see Chapter 13). Bedside echocardiography (ECHO) offers noninvasive, real-time, yet non-continuous assessment of CO in addition to other important parameters such as myocardial contractility, estimates of preload and afterload, etc. (see Chapters 9, 10, 11, and 12) The precision of echocardiographically estimated cardiac output is within the acceptable range for technology utilized for clinical applications, albeit approximately ±30%.19 It has become an integral part of routine bedside neonatal assessment20–22; however, to ensure accurate and reliable measurements, it requires appropriate training and sufficient practice.23,24 More so, a number of important limitations of ECHO need to be accounted for when assessment of systemic circulation is performed at the bedside. Unlike in older children and adults when left ventricular output (LVO) can be used as a surrogate of systemic blood flow with confidence, transitional changes of the cardiovascular system in a neonate may significantly affect LVO as it will no longer only represent systemic blood flow. For example, in the presence of a hemodynamically significant PDA (hsPDA) when substantial left-to-right shunting takes place, the resultant increase in LVO represents both systemic and ductal (pulmonary) blood flow. Relying on LVO in such cases will lead to overestimation of true systemic blood flow that can, in reality, be either within the normal range or decreased. Right ventricular output (RVO) has also been utilized as an assessment of systemic perfusion. However, with significant left atrial overload from pulmonary over-circulation in the presence of a hsPDA, left-to-right atrial shunting via the patent foramen ovale (PFO) will result in an increased RVO.25,26 Thus RVO in such cases reflects both systemic venous return and trans-atrial left-to-right flow through the PFO. Therefore a normal or increased LVO or RVO in the presence of hsPDA does not ensure adequate systemic blood flow. However, it is important to point out that a low LVO or RVO in the presence of a hsPDA indicates low systemic blood flow. Alternatively, superior vena cava (SVC) flow, which is not affected by the presence of either interatrial or transductal shunts, has been studied and proposed as a surrogate of systemic perfusion.27 While not without its own limitations, decreased SVC flow has been associated with adverse short- and long-term outcomes.2,4 Finally, and of utmost importance, in any situation when congenital heart disease is suspected clinically or has been prenatally diagnosed, a comprehensive anatomic echocardiography evaluation must be performed and then interpreted by a pediatric cardiologist. Electrical biosensing technologies (EBT) enable continuous and noninvasive assessment of CO, utilizing the changes in bioimpedance or bioreactance during the cardiac cycle (Chapter 12). It should, however, be noted that the interchangeability of EBT with transthoracic echocardiography is poor and evidence of accurate trending ability is lacking.28,29 Many other modalities of cardiac output monitoring are described in Chapter 12. Near-infrared spectroscopy (NIRS) utilizes the principle of the different absorbency patterns of near-infrared light by oxyhemoglobin and deoxyhemoglobin to measure tissue oxygenation index (TOI) or regional tissue oxygen saturation (rSO2). Thus NIRS also provides information on tissue oxygen extraction in vital and non-vital organs. Therefore it allows the indirect assessment of organ blood flow noninvasively, and in a continuous manner. Indeed, with caution, it can be used as a surrogate of organ blood flow30,31 provided that arterial oxygen saturation (SpO2), the metabolic rate for oxygen, the ratio of arterial-to-venous blood flow in the target organ, and hemoglobin concentration during the assessment remain stable. A growing body of evidence supports the clinical use of NIRS in neonates, particularly for assessment of cerebral tissue oxygen saturation (CrSO2). A number of studies have reported on the changes in CrSO2 in preterm and term neonates during transition32–34 and investigated the association between changes in CrSO2 and adverse short- and long-term outcomes.35–38 Of note is the fact that earlier studies have important limitations due to the non-continuous assessment of CrSO2. This methodological problem has, for instance, resulted in contradicting reports on the association between changes in CrSO2 and the development of peri-/intraventricular hemorrhage (P/IVH) in preterm neonates (see Chapters 7 and 8). Both increased mean CrSO2 along with decreased mean fractional tissue oxygen extraction (FTOE)35 and, conversely, decreased CrSO2 along with increased FTOE values37 have been reported in preterm neonates that developed P/IVH during the first few postnatal days compared to controls. When the systemic and cerebral hemodynamic changes were investigated in extremely preterm neonates using intermittent assessment of cardiac function by ECHO and mean velocity in the middle cerebral artery (MCA) by Doppler ultrasound along with continuous CrSO2 monitoring, the identified early pattern of hemodynamic changes in preterm neonates later affected by P/IVH suggested a plausible pathophysiologic explanation for such discrepancies in the reported findings (Chapter 7).36 Affected neonates demonstrated initial systemic and cerebral hypoperfusion, followed by improvement in both systemic and cerebral blood flow as indicated by the increase in CO, MCA mean velocity, and CrSO2 during the subsequent 20–44 hours and preceding detection of P/IVH. Of note, partial pressure of arterial carbon dioxide (PaCO2) also increased prior to detection of P/IVH. These observations support the hypoperfusion-reperfusion hypothesis as the major hemodynamic pathophysiological factor in the development of P/IVH and underscore the advantages of continuous rSO2 monitoring in neonates using NIRS technology. Furthermore, findings of several recent studies investigating the changes in CrSO2 in conjunction with other hemodynamic parameters and brain functional activity have enabled the assessment of cerebral autoregulation dynamics and its potential clinical implications in preterm and term neonates under different conditions (see below). The role of cerebral NIRS as a routine monitoring tool in extremely preterm infants is currently being evaluated in the SafeBoosC-III trial. The observations on the gender-specific differences in vascular tone regulation and peripheral perfusion in preterm and term neonates and the findings that, in patients with sepsis or anemia, changes in microcirculation precede the changes in other hemodynamic parameters or laboratory values indicate the importance of the assessment of microcirculation in the overall evaluation of the neonate. Perfusion index (PI), defined as the ratio of the pulsatile and non-pulsatile components of the photoelectric plethysmographic signal of pulse oximetry, has been used as a marker of peripheral perfusion.39–43 It is an appealing measurement as it is noninvasive and continuous and theoretically should provide insight into the circulatory status as it is expected to be affected by a reduction in stroke volume. The signal is readily available at a low cost in many NICUs, with good signal quality in the first 24 hours and without the need for additional monitoring equipment.44 Interpretation of crude PI values at the bedside is complex due to its dependency on different clinical variables such as positioning of the sensor, the presence of a hsPDA, influence of ventilation strategy, and both gestational and postnatal age.45 Although the raw PI signal might not be considered informative during early transition in both preterm and term neonates,46–48 multiple features of a processed PI sub-signal in the first 72 hours of life are associated with adverse outcome in extremely preterm infants.49,50 There is also strong evidence that preductal values of PI are strongly correlated with low cardiac output states in preterm infants, which advocates the use of the PI signal in trend monitoring.51 In addition, there is emerging evidence that PI can detect early microcirculatory changes associated with late onset in preterm infants.52 Whether the PI can be used reliably in the clinical setting for monitoring of perfusion in patients with hsPDA remains uncertain. While, compared to neonates with a non-hsPDA or no PDA, some studies reported a difference in pre- and post-ductal PI values in patients with a hsPDA during the early transitional period,53 others reported no effect of ductal flow and/or its persistence on PI.54 Moreover, the presence of the reported pre- and post-ductal gradient in both preterm55 and term neonates resolved by postnatal day 5.56 More recently, Gomez-Pomar identified that the mean ΔPI, mean pre-PI, and the ΔPI variability were lower in infants with a PDA, many of whom had echocardiography studies performed after 5 days of postnatal life.57 PI has also been evaluated as a tool to help identify critical congenital heart disease, in particular for infants with left heart obstruction.58 Several other methods are currently available to assess peripheral perfusion and the microcirculation. They include but are not limited to orthogonal polarization (OPS) and side-stream dark-field (SDF) imaging,59–64 laser Doppler flowmetry,65–68 and visible light technology.69,70 Videomicroscopy techniques (OPS and SDF) allow direct visualization of the microcirculation. However, their bedside use in neonates has been limited to intermittent assessments of peripheral perfusion rather than continuous monitoring. While continuous recording of the images can be done, real-time assessment and interpretation remain challenging at this point, with motion and pressure artifacts posing a significant problem. A newer technique, incident dark field imaging (IDF), appears to be superior to SDF in image quality and accuracy of assessment of the microcirculation in preterm neonates but also bears the limitation of non-continuous evaluation.71 Laser Doppler flowmetry, on the other hand, offers the capability of continuous monitoring and has been also used in the neonatal population. The main limitations of the technology include inability to evaluate absolute flow properties and thus only allowing assessment of the relative changes in flow over time, low temporal resolution requiring measurement times of ∼1 minute, and technical challenges (motion artifacts) to maintain proper probe position on the patient for an extended period of time. A newer laser-based technology, laser speckle contrast imaging (LSCI),72–75 addresses the issue of temporal resolution with significantly faster measurement times. However, similar to laser Doppler flowmetry, it provides only relative measurements of flux. In addition, LSCI has not been studied in the neonate yet. Assessment of the microcirculation utilizing visible light technology has been reported.76 However, little is known about its utility in the neonatal population.77 Table 14.1 summarizes the tools available for bedside monitoring of the various physiologic parameters discussed in this section. Physiologic parameters of systemic blood flow (BP and CO) and oxygenation, carbon dioxide production and elimination, regional (organ) and peripheral (microcirculation) blood flow, and organ (brain) function (aEEG) with corresponding assessment tools are listed that can be monitored at the bedside along with the indirect methods used in clinical practice to evaluate perfusion and organ function. Data acquisition can be continuous [C], intermittent [I], or both [C/I]. Adapted from Azhibekov et al., 2014.31 A growing body of evidence underscores the importance of, and the need for, incorporating multiple physiologic parameters when evaluating the hemodynamic status of neonates. Increasingly, investigators have combined data from different monitoring tools to improve their diagnostic and prognostic value. Such an approach frequently provides valuable insights into the underlying physiological processes as well as the pathophysiology of the cardiovascular compromise that would not be possible with monitoring tools employed in isolation.78–81 As an example, cerebral autoregulation, that is, the ability of the brain to maintain cerebral blood flow during fluctuations of blood pressure within a certain blood pressure range (Chapters 2, 3, and 8),82 has been studied using the interaction between the two aforementioned parameters (blood pressure and cerebral blood flow). Significant advances have been made in both studying cerebral autoregulation83 and improving our prognostic capability in preterm neonates,84 especially in those at risk for the development of P/IVH and in term neonates with hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia.85,86 Inclusion of continuous monitoring of PaCO2, the most important and powerful regulator of cerebral blood flow,87–89 has the potential to provide additional information about the complex interactions between PaCO2, cerebral blood flow, and other hemodynamic parameters. This is of importance as significant alterations in PaCO2 (both hypo- and hypercapnia) have been associated with adverse short- and long-term outcomes.36,90,91 Currently, clinical and conventional physiologic data are collected and documented manually in the patient’s chart or recorded automatically from the bedside monitors to the patient’s electronic medical records (EMR). This information, however, is typically documented on an hourly or bi-hourly basis only and may be subject to human error. These intervals are probably inadequate for appropriate monitoring of the rapid and dynamic changes characteristic of the cardiovascular system. To be able to accurately assess the overall hemodynamic status, identify relevant changes in a timely manner, and understand the intricate interplay among the different hemodynamic parameters, these data need to be collected at much higher frequencies (sampling rates) and be time-stamped to other relevant clinical events. The development of comprehensive hemodynamic monitoring systems enables real-time, simultaneous, and continuous collection of physiologic data in a reliable and comprehensive manner for subsequent analysis and assessment of the complex interactions among multiple hemodynamic parameters that may change significantly in a matter of seconds or minutes. Such systems include various monitoring tools, enabling concomitant evaluation of both systemic and regional perfusion and oxygen delivery and other physiologic parameters that play a role in cardiovascular regulation and adaptation, along with monitoring the functional status of specific organs (Figure 14.1). Advances in biomedical technology and computer science have improved the capabilities of comprehensive monitoring systems to collect and store increasing sets of complex physiometric data. Training computer algorithms using hemodynamic high-frequency monitoring data, in combination with nursing observation parameters and patient history details, has the potential to uncover disease-associated patterns in clinical data that are not (always) visible to the human eye. Machine learning techniques could aid clinicians in targeting treatment in an individualized manner, and artificial intelligence software solutions will become available over time. For these models to be developed correctly and to be deemed clinically relevant, the medical and neonatal community urgently needs to develop a strategy for the critical appraisal and assessment of the technology aids that use modeling on time series data. However, the caveats regarding their accuracy, feasibility, reliability, and need for validation across various subpopulations must be emphasized.92,93 Finally, and as previously discussed, the utility of the monitoring systems is determined by the comprehensiveness of the monitored hemodynamic parameters. A previously described hemodynamic monitoring “tower” developed by some of the authors91,92 was the first step in the process to enable practical, continuous, and simultaneous monitoring and acquisition of neonatal hemodynamic data at high sampling rates in real time, initially designed for research applications. It integrates conventionally used technologies to continuously monitor heart rate, blood pressure, SpO2, transcutaneous CO2 tension (TCOM), and respiratory rate with other technologies such as EBT for beat-to-beat measurements of SV and CO and NIRS for continuous monitoring of rSO2 changes in vital (brain) and non-vital (kidney, intestine, and/or muscle) organs. The “tower” incorporates these various parameters onto a comprehensive patient monitor using conventional or VueLink modules (Phillips, Palo Alto, CA). The continuous stream of measurements is then acquired through the analog output of the monitor via the use of an analog-to-digital converter and data-acquisition system onto a laptop computer. A motion-activated camera with the same time stamp is used to capture the bedside events that could affect the accuracy and interpretation of the collected data. This functionality aids in differentiating between true fluctuations of physiologic data versus equipment malfunction (lead disconnection, electrode/optode displacement) or other potential artifacts related to provision of routine clinical care and procedures. Another unique advantage of the “tower” is its ability to operate as a mobile, stand-alone unit that could be utilized at any bedside, space permitting. With the increasing recognition of the importance of comprehensive monitoring in intensive care settings in the last decade, several central data collecting systems have become available (for example, Sickbay and Etiometry). These platforms allow for display of interaction of various physiological (including hemodynamic) parameters in graphic formats at bedside. Collection of all the data with a single monitoring device enables automatic data synchronization and avoids the need to match different time stamps so that simultaneous minute-to-minute changes and interactions between various parameters can be reliably analyzed. Relevant clinical events such as fluid bolus administration, titration of vasoactive medications or treatment of a PDA, transfusion of blood products, intubation/extubation, and change in respiratory support are manually documented by the bedside nurse on a dedicated flowsheet. Figure 14.2 demonstrates an example of a 1-hour period of processed patient data that was acquired using the hemodynamic monitoring “tower” of a preterm infant with respiratory failure on volume-guarantee ventilation. Figure 14.3 demonstrates the hemodynamic changes that were observed during various events related to routine patient care and extubation. Retrospective review of the video data captured by the motion-activated camera during the study period enabled identification and accurate time-stamping of these events. Without the concomitant video component, proper interpretation of the physiologic data would have been challenging, if not impossible.
Chapter 14: Comprehensive, real-time hemodynamic monitoring and data acquisition: An essential component of the development of individualized neonatal intensive care
Introduction
Limitations of conventional monitoring
Assessment of systemic and regional blood flow
Systemic blood flow
Organ blood flow
Peripheral perfusion and microcirculation
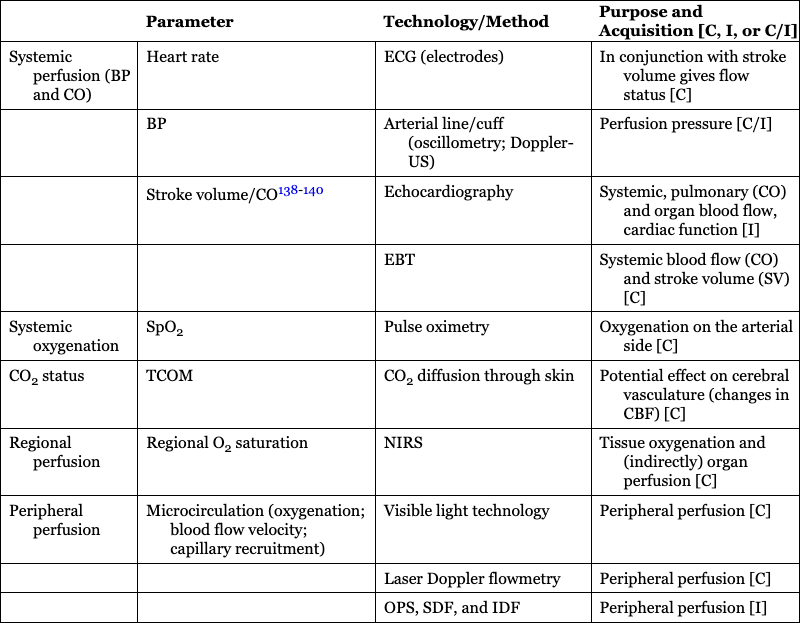
Parameter
Technology/Method
Purpose and Acquisition [C, I, or C/I]
Systemic perfusion (BP and CO)
Heart rate
ECG (electrodes)
In conjunction with stroke volume gives flow status [C]
BP
Arterial line/cuff (oscillometry; Doppler-US)
Perfusion pressure [C/I]
Stroke volume/CO138–140
Echocardiography
Systemic, pulmonary (CO) and organ blood flow, cardiac function [I]
EBT
Systemic blood flow (CO) and stroke volume (SV) [C]
Systemic oxygenation
SpO2
Pulse oximetry
Oxygenation on the arterial side [C]
CO2 status
TCOM
CO2 diffusion through skin
Potential effect on cerebral vasculature (changes in CBF) [C]
Regional perfusion
Regional O2 saturation
NIRS
Tissue oxygenation and (indirectly) organ perfusion [C]
Peripheral perfusion
Microcirculation (oxygenation; blood flow velocity; capillary recruitment)
Visible light technology
Peripheral perfusion [C]
Laser Doppler flowmetry
Peripheral perfusion [C]
OPS, SDF, and IDF
Peripheral perfusion [I]
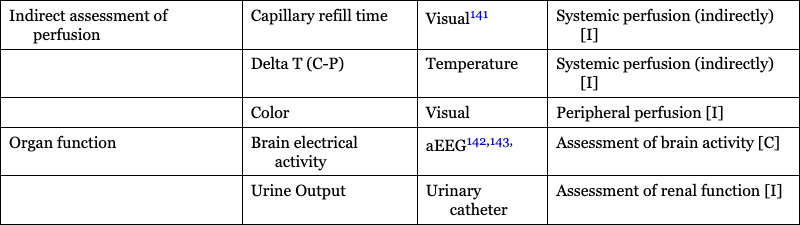
Indirect assessment of perfusion
Capillary refill time
Visual141
Systemic perfusion (indirectly) [I]
Delta T (C-P)
Temperature
Systemic perfusion (indirectly) [I]
Color
Visual
Peripheral perfusion [I]
Organ function
Brain electrical activity
aEEG142,143,
Assessment of brain activity [C]
Urine Output
Urinary catheter
Assessment of renal function [I]
Comprehensive monitoring systems
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Obgyn Key
Fastest Obstetric, Gynecology and Pediatric Insight Engine
