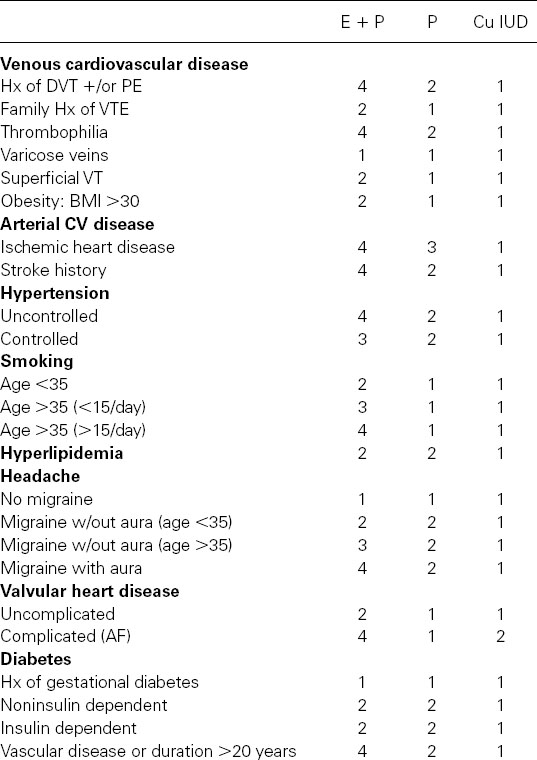
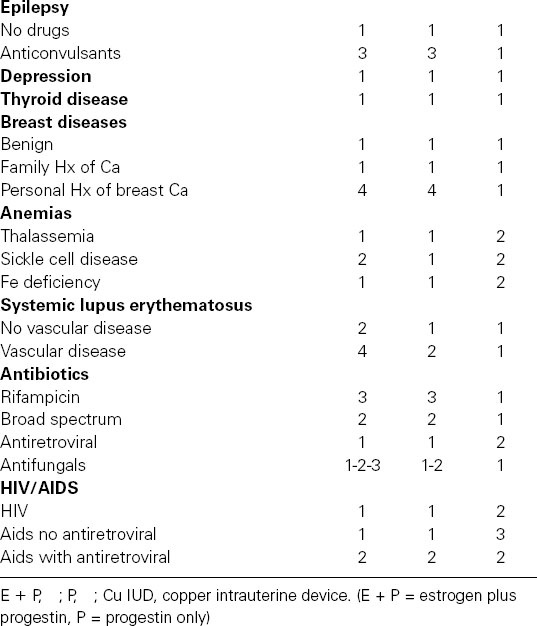
Table 107.1 Medical eligibility criteria for contraception adapted from WHO guidelines (Category 1: No contraindications, Category 2: Benefits outweigh risks, Category 3: Risks outweigh benefits, Category 4: Contraindicated)
Initiating oral contraceptive therapy
In deciding whether the pubertal, sexually active girl should use steroid contraception, the clinician should be more concerned about compliance than possible physiologic harm. Provided the postmenarchal girl has demonstrated maturity of the hypothalamic-pituitary-ovarian axis by having at least three regular, presumably ovulatory cycles, it is safe to prescribe hormonal contraceptives without being concerned about causing permanent alterations of hypothalamic-pituitary function. One need not be concerned about accelerating epiphyseal closure in postmenarchal females. Their endogenous estrogens have already initiated the process a few years before menarche, and the contraceptive steroids will not hasten it. These agents can also be prescribed for women with oligomenorrhea, especially those with polycystic ovarian syndrome and dysfunctional bleeding due to anovulation. COCs will inhibit testosterone secretion and, by increasing levels of sex hormone-binding globulins, will increase the binding of biologically active testosterone, thus reducing the manifestations of hyperandrogenism. In both these conditions, COCs, the patch and ring will produce regular uterine bleeding without excessive blood loss. The progestin component of these agents will reduce the incidence of endometrial hyperplasia due to the effect of unopposed estrogen in these anovulatory women.
Following pregnancy
Ovulation occurs sooner after a spontaneous or induced abortion, usually between 2 and 4 weeks, than after a term delivery, when ovulation is usually delayed beyond 6 weeks but may occur as early as 4 weeks in a woman who is not breastfeeding.
Thus after spontaneous or induced abortion of a fetus of less than 12 weeks’ gestation, steroid contraception should be started immediately to prevent conception occurring with the first ovulation. For women who deliver after 28 weeks and are not nursing, use of these agents should be initiated 2–3 weeks after delivery. If the termination of pregnancy occurs between 12 and 28 weeks, estrogen-containing contraceptive should be started 1 week after the end of the pregnancy. The reason for the delay in the latter instances is that the normally increased risk of thromboembolism occurring post partum may be further enhanced by the hypercoagulable state associated with estrogen-containing contraceptives. Because the first ovulation is delayed for at least 4 weeks after a term delivery, there is no need to expose the woman to this increased risk.
It is probably best for women who are nursing not to use estrogen-containing contraceptives, as their use, even with the low estrogen dose formulations, has been shown to diminish the amount of milk produced, as estrogen inhibits prolactin’s action on the breast. Women who are breastfeeding every 4 hours, including at night, will not ovulate until 6 months after delivery if they do not menstruate and thus do not need contraception before that time. Once any supplemental feeding is introduced after 4 weeks post partum, ovulation can resume promptly. Since only a small percentage of full breastfeeding women will ovulate within 6 months post partum as long as they continue full nursing and remain amenorrheic, there is no need for additional contraceptive use. Progestin-only methods of contraception, in contrast to the estrogen-containing products, do not diminish the amount of breast milk and are effective in this group of women. However, a small portion of these synthetic steroids have been detected in breast milk. The long-term effects (if any) of these progestins on the infant are not known, but none has been detected to date.
All women
At the initial visit, after a history and physical examination have determined that there are no medical contraindications for COCs, patch and ring, the woman should be informed about their benefits and risks. For medicolegal reasons, it is best either to use a written informed consent signed by the woman or note on the woman’s medical record that the benefits and risks have been explained to her and that she has been told to read the patient package insert.
Type of formulation
In determining which formulation to use, it is best to prescribe initially a COC formulation with less than 50 μg of ethinyl estradiol, since these agents are associated with less cardiovascular risk, as well as fewer estrogenic side effects, than 50 μg estrogen-containing formulations. It would also appear reasonable to use oral formulations with the lowest dosage of a particular progestin because fewer progestogenic metabolic and clinical adverse effects would be associated with their use. The patch and ring are currently made with only a single amount and type of estrogen and progestin. The development of multiphasic formulations has allowed the total dose of progestin to be reduced compared with some monophasic formulations without increasing the incidence of breakthrough bleeding. However, several monophasic formulations have a lower total dose of progestin per cycle than the multiphasic formulations. In addition, the most recently developed progestins derived from levonorgestrel: desogestrel, norgestimate, and gestodene have less androgenic activity than the older progestins norethindrone and its acetates and levonorgestrel. The progestin drosperinone has antiandrogenic as well as antimineralocorticoid properties and formulations with this agent are approved to treat acne and premenstrual dysphoric disorder in women desiring contraception. Some other formulations are also approved to treat acne.
The Food and Drug Administration (FDA) has stated that the product prescribed should be one that contains the least amount of estrogen and progestin that is compatible with a low failure rate and the needs of the individual woman. Because few randomized studies have been performed comparing the different marketed formulations, until large-scale comparative studies are performed, the clinician must decide which formulation to use based on which have the least adverse effects among women in his or her practice. If estrogenic or progestogenic side effects occur with one oral formulation, a different agent with less estrogenic or progestogenic activity can be given. Oral formulations with 20 μg of estrogen and the same amount of progestin as those with 30 μg appear to cause more unscheduled bleeding. If unscheduled bleeding persists beyond a few months after initiation, a formulation with a higher amount of estrogen can be used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


