To compare children of different ages and gender, the height velocity is converted to a z-score:
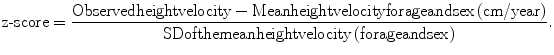
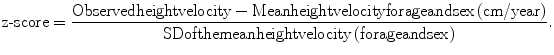
The z-score corresponds to the standard deviation (SD) of the child’s height velocity. Points are allocated to the number of standard deviations below normal, defined as greater than or equal to −1 SD.
The PCDAI has been evaluated in five cohorts of children with CD (Table 41.1) [19, 22, 23, 25, 26]. The demographic characteristics and distribution of disease found in these cohorts were similar to previously described pediatric CD populations. In a head-to-head comparison, Otley et al. [22] showed that the PCDAI was highly correlated with physician global assessment (PGA) (r = 0.86) higher than the CDAI (r = 0.77), the modified CDAI (r = 0.76) and the HBI (r = 0.72). Inter-observer reliability was demonstrated by concurrent calculation of the PCDAI by two independent gastroenterologists. In the largest study to date on the PCDAI, test–retest reliability on stable patients has been shown to be good [23]. Responsiveness to change in disease activity was demonstrated and the minimal clinically important change, to define “response,” was found to be at least 12.5 points [25] or >12 points in the larger study which used several methods to attain this “minimal important difference” corresponding to moderate change. Large change was associated with a change of at least 30 points [23]. The optimal PCDAI score representative of disease remission has been open to some discussion. The initial study found that a PCDAI score of less than or equal to 10 points discriminated active from quiescent disease. Other studies found that PCDAI scores of less than 10 and 15 points were more sensitive and specific to Hyams et al. [26] and Otley et al. [22], respectively. In the most recent study of 366 children, the best cut-off values were <10 points for remission, 10–27.5 for mild disease, 30–37.5 for moderate disease and 40–100 for severe disease. However, evaluating the accuracy of different values for remission, the authors recommend defining remission as <10 points or <7.5 without the height item. This yielded the best accuracy acknowledging that the growth item is irrelevant in adolescents who passed the growing-Tanner stages and that height typically improves several weeks or months after remission has been achieved (i.e. low responsiveness). This is problematic since the growth item contributes 20 points to the total score.
Table 41.1
Validity, reliability, and responsiveness of Pediatric Crohn Disease Activity Index
Instrument | Study population | Validity | Reliability | Responsiveness | ||
|---|---|---|---|---|---|---|
PCDAI Hyams et al. [19] | n = 131 prospective cohort | PCDAI to HBImod r = 0.81 | Inter-observer r = 0.86 | N/A | ||
PCDAI to PGA r = 0.80 | ||||||
Score cut-offs: | ||||||
No disease | 0–10 | 69% | ||||
Mild | 11–30 | Correct | ||||
Moderate/Severe | >30 | Classification | ||||
Otley et al. [20] | n = 81 prospective cohort | PCDAI to CDAI r = 0.86 PCDAI to PGA r = 0.86 | N/A | The difference in the PCDAI score between the two visits was highly correlated with the difference in the CDAI in 17 patients. No other responsiveness measures are provided and time of follow-up visit not specified | ||
PCDAI to HBI r = 0.84 | ||||||
Receiver operating curves to select PCDAI cut-offs for no versus mild disease: | ||||||
Sensitivity | Specificity | |||||
≤10 | 0.75 | 0.905 | ||||
<15 | 0.83 | 0.905 | ||||
Hyams et al. [22] | n = 181 from Pediatric IBD Collaborative Research Group Registry | Validation of previously defined score cut-offs: | N/A | Clinically significant change in PCDAI predictive of change in PGA = 12.5 points (sensitivity 0.87, specificity 0.73) | ||
Sensitivity | Specificity | |||||
No disease vs. mild: <10 | 0.81 | 0.68 | ||||
Mod/severe vs. mild: >30 | 0.71 | 0.83 | ||||
Kundhal et al. [21] | n = 25 and 63 (from two prospective cohorts) | N/A | N/A | Minimal clinically significant change in PCDAI predictive of PGA at 1-month follow-up = 12.5 points (sensitivity 0.83, specificity 0.92) | ||
n = 437 (from four prospective cohorts) | PCDAI to PGA r = 0.67 PCDAI to CRP r = 0.26 | n = 90 | The PCDAI showed good responsiveness to change (r = 0.54–0.83, distributional 0.8–1.4, diagnostic utility analyses AUC ROC 0.79–0.85); minimal important difference >12 points | |||
PCDAI to ESR r = 0.49 PCDAI to Alb r = −0.37 | ICC: 0.74–0.8 | |||||
PCDAI to Hb r = −0.40 PCDAI to Plat r = 0.58 | ||||||
The feasibility of the PCDAI is only moderate; in the registry of a pediatric IBD collaborative research group, only 47.6% of the registered visits had a valid PCDAI score, compared to 97.6% with the pediatric UC activity index (PUCAI) [27]. Similarly, data to complete the PCDAI from the ImproveCareNow registry were available in the charts of only 20% of 3643 clinical visits [28]. Besides the low feasibility of the index and the limitations imposed by the growth item, the inclusion of the perianal item is debated as it reflects a different concept than luminal disease activity. Finally, the PCDAI does not differentiate well moderate from severe disease activity.
Recently, the PCDAI was subjected to a mathematical weighting on 437 children with Crohn disease [29] (Appendix 1-3). The newly weighted PCDAI, termed wPCDAI, excluded three items shown to be redundant in reflecting disease activity: height velocity, abdominal examination and hematocrit. The score range of the wPCDAI is 0–125. In the validation cohort it had higher correlation with PGA and ESR than the original PCDAI (0.75 vs. 0.67 and 0.58 vs. 0.49, respectively). More importantly, the discriminant validity was much better with the wPCDAI version: it differentiated those in remission from active disease (area under the ROC curve 0.95) and, unlike the original PCDAI, differentiated moderate from severe disease (area under the ROC curve 0.87). Further validation studies are underway to determine if the wPCDAI can replace the PCADI in future clinical studies.
A number of abbreviated PCDAI instruments have been proposed to increase the feasibility of the PCDAI for use in retrospective chart reviews [24, 28, 30–32]. The abbreviated PCDAI (abbrPCDAI) retained the three history variables (abdominal pain, general well-being and stools per day), weight variable, abdominal exam and perirectal disease. Recently, a larger study presented a short version of the PCDAI (shPCDAI), excluding items with a low frequency of completion in a patient registry [28]. The difference between the shPCDAI and the abbrPCDAI is that the extraintestinal manifestation item has replaced the perianal item and new weights have been mathematically assigned to each item, reflecting their relative importance to PGA of disease activity. The exclusion of the lab items in both indices increased their feasibility but at the expense of reduced validity when compared head-to-head with the other PCDAI versions [24]. Nonetheless, these versions may be used in retrospective studies when not all the items required for the full index are available. A third abbreviated version, a modified PCDAI (modPCDAI), aims to provide a measure of disease activity in pediatric Crohn disease when only blood tests are available (e.g. in administrative databases). The modPCDAI includes the three laboratory items of the PCDAI (i.e. hematocrit, ESR and albumin) in addition to the CRP. If enough data are available, the other PCDAI versions are preferred as disease activity is not a concept which can be reflected solely by blood test results [32].
Perianal Crohn Disease
Perianal Crohn disease encompasses perianal skin tags, fissures, enterocutaneous fistulas and abscesses. It affects up to one-third of the CD population and it may be the only manifestation of the disease [33, 34]. Numerous clinical trials have been devised, primarily in adult populations, to determine the optimal management of this challenging condition.
There are two disease activity measures currently used in clinical trials to follow perianal CD activity: the perianal disease activity index (PDAI) and the Fistula Drainage Assessment (Appendices 2-1 and 2-2). The PDAI was developed and validated by Irvine and colleagues [35]. It contains five items, each scored 0–4, with higher scores representing more severe disease. In the validation cohort, it had moderate correlation with both physician and patient assessment of perianal disease activity (r = 0.72 and 0.66 respectively). As expected, it correlated poorly with the CDAI and HBI (r = 0.23 and 0.21 respectively) implying that perianal disease can present in the absence of other CD symptoms. The PDAI scores were reproducible in patients with stable disease over 4–8 weeks, implying test–retest reliability. Although the PDAI changed in patients who improved or deteriorated on repeat visits, robust and accepted responsiveness statistics were not presented. The second instrument, the Fistula Drainage Assessment, was introduced as part of a clinical trial of infliximab therapy for perianal CD [36]. This instrument simply defines response as a 50% decrease in draining fistulas and complete response as fistula closure or the absence of any draining fistulas. In that study, the Fistula Drainage Assessment was significantly lower in the treatment versus the control group, similar to the PDAI calculated simultaneously. These results have been replicated in other clinical trials [37–39]. Reliability of this subjective index was never formally assessed; therefore, it is recommended that two independent physicians score the index in future clinical trials. Moreover, in view of the questionable validity and responsiveness of these indices, and since no other similar measure is available, both should be calculated in clinical trials, until more data is available.
There is no unique PDAI for children with perianal CD. In order for the PDAI to be considered in children, the sexual dysfunction component should be modified to reflect more age appropriate issues such as participation in school and other social activities. Obviously, this modification requires appropriate validation and assessment of reliability and responsiveness before being used in pediatric studies. At present, the use of the Fistula Drainage Assessment definitions appears to be the preferred perianal outcome in pediatric clinical trials, as an anchor for physician assessment of disease activity.
Ulcerative Colitis Disease Activity Indices
The earliest classification of UC disease activity was a qualitative scale published by Truelove and Witts in 1955 (Appendix 3-1) [40]. This index defined remission, mild and severe disease. A score between mild and severe was considered as moderate. Because of this simple gradation and poor definition of moderate severity, significant ambiguity exists in defining change in disease activity with this index. Arbitrary quantitative indices have since been introduced, including the Powell-Tuck Index [41], the Mayo-Clinic Score [42], Rachmilewitz Index [43] and Lichtiger Score [44]. The first three include an endoscopic evaluation of the rectosigmoid as part of the global assessment. Their validation has therefore been largely a side product of clinical trials in which they have been used and developed. Seo and colleagues recently developed and evaluated an UC disease activity index [45, 46], weighted against the Truelove and Witts classification. The initial development process was biased towards severe disease since the investigators used a retrospective cohort of hospitalized patients. It is not surprising, therefore, that the index is heavily weighted toward three (of five) lab items which may be more important in severe disease. This index has not gained wide use despite its multi-step development. Walmsley and colleagues developed a Simple Clinical Colitis Activity Index that removed all laboratory parameters [47]. It correlated highly with both the Powell-Tuck Index and Seo index. However, this index is not commonly used, perhaps because it was aimed only for initial assessment by non-specialist physicians as a guide for the need to seek further medical advice. Recently, the Endoscopic Clinical Correlation Index (ECCI) was developed prospectively in 137 adults with UC undergoing full colonoscopy [48]. Items were chosen based on their ability to predict endoscopic outcome. The ECCI highly correlated with the endoscopy colitis score (r = 0.81) slightly higher than the Seo, Truelove and Witts, Powell-Tuck and Walmsley’s simple colitis index. However, these correlations were obtained from the derivation cohort only and not from a separate validation cohort. Moreover, the reliability and responsiveness of the index were not assessed. In a prospective head-to-head study in adults of all non-invasive UC disease activity indices, the Walmsley index and PUCAI (see below) were best in assessing disease activity [49] when compared to a number of parameters including the full Mayo score and PGA. At this point in time, however, there is no prominent disease activity index, such as the CDAI for studies in adults with Crohn disease, for clinical trials in adults with ulcerative colitis (Appendix 3-2).
Unlike Crohn disease, UC has a more homogenous presentation and it is believed that most patients in remission have a normal, or near normal, endoscopic appearance. A position paper on the selection of outcomes in adult UC clinical trials recommended that the primary endpoint for therapeutic trials should be induction of remission (and exacerbation in maintenance studies), confirmed by an endoscopic assessment of the intestinal mucosa [50]. However, endoscopic assessment of disease activity has several major limitations. First, endoscopic scoring depends on the subjective assessment of mucosal properties with very low inter-observer reliability [51]. Second, endoscopic appearance lags after clinical improvement, thereby underestimating response to treatment [52]. Third, endoscopic remission usually starts in the ascending colon and progresses distally where the rectosigmoid is the last part to improve. Thus, limited sigmoidoscopy may not reflect response to treatment especially in children in whom extensive disease is the most common phenotype. Finally, although mucosal healing has been convincingly shown to predict favourable clinical outcome in UC [53–55], it is yet to be proven that endoscopy is superior to clinical judgement for assessing mucosal healing in general. Indeed, in the combined ACT cohorts (466 adults with UC treated with either infliximab or placebo), endoscopic healing had no predictive value among the subset of patients with clinical remission after 8 weeks of therapy [53].
Endoscopy in children requires sedation or general anaesthesia. Therefore, the need for repeat endoscopic assessment can deter children and their caregivers from participating in clinical trials. As it does not seem justified to recommend endoscopic assessment solely for research purposes, the PUCAI was developed omitting endoscopy variables (Appendix 3-3) [56]. The PUCAI was validated on a prospective cohort of 48 children undergoing full colonoscopy, wherein it showed very good to excellent correlation with PGA (r = 0.91), colonoscopic appearance (r = 0.77) and the adult reference index, the Mayo Clinic Score (r = 0.95). Correlations were higher than two non-invasive adult indices, the Seo and Lichtiger indices, calculated concurrently. Inter-observer and test–retest reliability were excellent (ICC = 0.95). The PUCAI differentiated well the categories of disease activity of none, mild, moderate and severe judged by a physician’s global assessment (areas under the ROC curves of >0.97). Responsiveness was shown to be high at repeated visits of 74 children. Remission was defined as a PUCAI score of <10, and the minimal clinically important difference as a change of at least 20 points (area under the ROC curve of 0.97). The laboratory items did not improve the validity or responsiveness of the PUCAI and thus were removed, making it attractive for pediatric use.
Since its introduction, the good performance of the PUCAI has been replicated in multiple independent cohorts [49, 57–59]. The cut-off values for remission and the different categories of disease activity have been replicated in two different studies [27, 49]. The PUCAI has been proven feasible on 215 children with UC from a prospectively maintained Pediatric registry wherein its predictive validity has been demonstrated by showing that children requiring escalation of medical therapy had higher PUCAI scores than those who did not. The correlation of the PUCAI with PGA in this cohort was 0.9. In two independent cohorts of children requiring admission for intravenous treatment of corticosteroids for UC exacerbations, the PUCAI has shown predictive validity, accurately identifying children who will require treatment escalation to second line medical therapy or colectomy, both by discharge and up to one year post discharge [57, 58]. In this setup, the PUCAI has shown to have superior predictive validity to five faecal biomarkers, including calprotectin [60, 61].
The PUCAI has since been reported in several pediatric clinical studies including two randomized controlled trials. In the T72 trial evaluating the effectiveness of infliximab in pediatric UC, the PUCAI determined week 8 remission rate was 33%, identical to the rate of complete mucosal healing found by sigmoidoscopy [62]. Similarly, the week 12 remission rate in a clinical trial evaluating beclomethasone 17,21-dipropionate in children with UC was similar whether determined by sigmoidoscopy or the PUCAI [63].
Gastrointestinal Endoscopy Indices
Crohn Disease
Assessment of gastrointestinal mucosal disease is important in CD research as mucosal healing has been associated with better long-term outcomes [64]. Two groups have developed standardized approaches to endoscopy findings in Crohn disease. The first designed the Crohn disease endoscopic index of severity (CDEIS) by incorporating endoscopic findings, previously shown to have high inter-rater reliability [65], into a regression model using the PGA of endoscopy severity as the dependent variable [66] (Appendix 4-1). The index was found to have high inter-rater reliability (r = 0.96) and was highly correlated with the physician endoscopy assessment in an independent cohort with Crohn disease (r = 0.81). It has subsequently been used in multiple clinical trials evaluating endoscopic endpoints [67–69]. However, it has been criticized for its complexity. Thus, Daperno and colleagues developed the Simplified Endoscopic Activity Score for Crohn disease (Appendix 4-2) [70]. The SES-CD had high inter-rater reliability (ICC = 0.98) and was highly correlated with the CDEIS (r = 0.92). The CDEIS was similarly found to have high inter-rater reliability (ICC = 0.91) in their study. Lower correlations were found between both the SES-CD and CDEIS and other parameters of disease activity including the CDAI (0.39 and 0.36 respectively) and C-reactive protein (r = 0.47 and 0.45 respectively) confirming that in Crohn disease, mucosal findings do not necessarily reflect a patient’s clinical status.
There is no standardized endoscopic instrument for pediatric CD. However, there is no evidence that endoscopic characteristics differ in children. Although the CDEIS is the most widely employed instrument in adult clinical trials, the SES-CD seems to be a valid alternative to its more complicated counterpart. The use of either instrument in pediatric studies should be supplemented with a physician global endoscopy assessment until further assessment in pediatric CD is available.
Post-operative Crohn Disease Endoscopic Assessment
After intestinal resection, recurrence of CD is almost inevitable when patients are followed-up long-term, and it usually occurs at the site of the surgical anastomosis [71]. Endoscopic recurrence precedes clinical recurrence; at 1-year follow-up, 70% of patients had endoscopic recurrence but only 20% were symptomatic [72]. Therefore, endoscopy is an important outcome in clinical trials assessing post-operative interventions in Crohn disease. Rutgeerts and colleagues [72] proposed a scoring system for recurrent endoscopic disease at the surgical anastomosis based on their clinical experience (Appendix 4-3). The Rutgeerts endoscopic scoring system has been incorporated into numerous clinical trials and although it is quite subjective, higher Rutgeerts scores consistently predict a more severe clinical course [72–74]. However, as the reliability of this index has never been formally assessed, it would be prudent for two independent physicians to score the index in future clinical trials, until more data is available.
Ulcerative Colitis Endoscopic Assessment
No endoscopic index has been rigorously developed and evaluated in children. The lack of an evaluated measure is concerning in view of the potential low reliability of some endoscopic assessments [75]. Baron and colleagues found only 66% agreement on a subset of endoscopic findings in adults with proctocolitis [75]. Specifically, spontaneous bleeding, friability and the presence of ulcers were found to have greater than 66% agreement only if classified as present or absent. If characteristics of these variables, for example, the extent of friability, were added to the endoscopic assessment then the agreement was poor on all items. Recently, moderate to good agreement (k > 0.39) was observed among four experienced colonoscopists for 10 of 14 signs or patterns of mucosal appearance in ulcerative colitis [76]. The agreement was poor, however, for mild and moderate disease activity. Therefore, inter-observer variability of endoscopy findings in ulcerative colitis requires further evaluation.
Although there are no standardized endoscopic assessment instruments available for ulcerative colitis, repeated endoscopic assessment is standard practice in adult UC clinical trials [77]. As justified above, a similar standard may not be feasible or essential in pediatric trials.
Quality of Life Instruments
Both adults and children diagnosed with IBD are at increased risk of emotional problems and decreased social functioning [78, 79]. Thus, quality of life (QOL) assessment has been increasingly recognized as an important and independent clinical outcome in IBD research. There are two types of QOL instruments, generic and disease specific. The choice of instrument depends on the clinical question. Generic QOL instruments are preferred when comparing QOL in different diseases. However, for assessing only IBD populations, a disease specific QOL instrument should be used. A thorough discussion of QOL instruments available for pediatric IBD research is found in chapter 45.
Summary of Clinical Outcome Measures
Various instruments are available to measure clinical outcomes in pediatric inflammatory bowel disease (Table 41.2). Valid pediatric clinical indices and QOL measures exist for both UC and CD. Endoscopic scores, however, have not yet been evaluated in children. The evaluation of health related indices is an ongoing process and therefore, the development of new indices and the re-evaluation of the performance of existing indices will continue to be explored in different clinical and research settings.
Table 41.2
Clinical indices for research in pediatric inflammatory bowel disease
Clinical trial outcome | Instrument | |
|---|---|---|
Crohn disease | Ulcerative colitis | |
Disease activity index | Physician global assessment PCDAI | Physician global assessment PUCAI |
Perianal disease activity index | Fistula Drainage Assessment | N/A |
Endoscopic scores | CDEIS (assessed only in adults) | Numerous non-validated indices |
SES-CD (assessed only in adults) | ||
Quality of life instruments Generic | Multiple (e.g. PedsQL, Child QOL questionnaire) | Multiple (e.g. PedsQL, Child QOL questionnaire) |
Disease specific | IMPACT-III | IMPACT-III |
Appendix 1
Appendix 1-1: Crohn Disease Activity Index (CDAI) and Harvey Bradshaw Index (HBI)
Instrument items | CDAI [13] | HBI [16] |
|---|---|---|
Clinical signs and symptoms | ||
Stools | Sum of liquid/soft stools (×2) | No. of liquid/soft stools |
Abdominal pain | Sum of daily score of 0–3 (×5) | 0–3 |
General well-being | Sum of daily score of 0–4 (×7) | 0–4 |
No. of complications | 1/item (6 categories) (×20) | 1/item (8 categories) |
Physical exam | ||
Abdominal mass | 0–2 (×10) | 0–3 |
Weight | 1-(wt/standard wt) × 100 | – |
Laboratory variables | Hct (×6): 47—Hct (male) | – |
42—Hct (female) | ||
Other | Use of anti-diarrheals: 0–1 (×30) | – |
Score cut-off for disease activity | Remission <150 | Remission ≤4 |
Mild 150–300 | Relapse >4 | |
Moderate 300–450 | ||
Severe >450 | ||
Comments | Clinical symptoms based on 7-day diary | Clinical symptoms based on previous 24 h |
Appendix 1-2: Pediatric Crohn Disease Activity Index [19]
History (Recall, 1 week) | |||||||
Abdominal Pain | Score | ||||||
0 = None | 5 = Mild: Brief, does not interfere with activities | 10 = Moderate/Severe: Daily, longer lasting, affects activities, nocturnal | ______ | ||||
Patient Functioning, General Well-Being | Score | ||||||
0 = No limitation of activities, well | 5 = Occasional difficulty in maintaining age appropriate activities, below par | 10 = Frequent limitation of activity, very poor | ______ | ||||
Stools (per day) | Score | ||||||
0 = 0–1 liquid stools, no blood | 5 = Up to 2 semi-formed with small blood, or 2–5 liquid | 10 = Gross bleeding, or ≥6 liquid, or nocturnal diarrhoea | ______ | ||||
Laboratory | |||||||
Hematocrit (HCT) | Score | ||||||
<10 years: | 11–14 years (Male): | ||||||
0 ≥ 33% | 2.5 = 28–32% | 5 ≤ 28% | 0 ≥ 35% | 2.5 = 30–34% | 5 ≤ 30% | ||
11–19 years (Female): | 15–19 years (Male): | ||||||
0 ≥ 34% | 2.5 = 29–33% | 5 ≤ 29% | 0 ≥ 37% | 2.5 = 32–36%
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||