(1)
Bordeaux, France
Classic ovulation stimulation involves three successive steps:
1.
Stimulating follicular growth with a goal of producing one to three fully-developed follicles
2.
Triggering ovulation to induce follicular rupture and extrusion of the oocyte(s) that have resumed meiosis and become fertilizable
3.
Establishing a corpus luteum capable of sustaining implantation and development of the early embryo
It should be noted at this point that most of the effort involving protocol development have essentially concerned the stimulation step. However it is at least as important that the two other steps should progress in a harmonious way that facilitates chances for a successful pregnancy in the selected indication, be it correction of ovulation abnormalities, restoration of ovulation, improvement of cervical mucus, or preparation for an intra-uterine insemination.
9.1 Objectives
The stimulation itself should work toward two principal goals:
To mimic or adjust the normal menstrual cycle. Stimulation of monofollicular development consists of provoking or facilitating growth of one single follicle in the same manner normally accomplished by physiologic menstrual cycle, whereas a paucifollicular stimulation seeks the growth of two or three follicles. In order to mimic or alter slightly the natural cycle, one should strictly apply the rules already established by nature, i.e., to apply a strategy of gonadotropin administration that follows the basic principles of identifying an FSH threshold and window. Manipulating these parameters should attain the chosen objective, whatever the beginning situation may have been.
To avoid complications: Insufficient or inadequate stimulation may result in a defective ovulatory process (e.g., abnormalities of oocyte maturation, follicular rupture, or corpus luteum function) that can paradoxically decrease the patient’s chances for successful pregnancy during the treated cycle. This outcome should be considered as a true complication despite the absence of “symptoms.” A succession of treatment failures make it tempting for patients to consider without due cause the more difficult road to assisted procreation, when the reality was simply a poor management of the ovarian stimulation.
Excessive stimulation is much easier to recognize because it is more symptomatic, exposing the patient to risks for ovarian hyperstimulation, multiple pregnancy, or both. The risks for these untoward results are of course greater in paucifollicular than monofollicular stimulations.
Ovarian hyperstimulation might occur only when development of the leading follicle(s) evolves in concert with numerous smaller follicles (<10 mm), a situation that can be easily discovered by ultrasound and plasma estradiol levels that rise abnormally high for one to three mature follicles.
Multiple pregnancy can only occur when a dominant and additional secondary follicles develop together, and become capable of growing and ovulating during the activity lifespan of the hCG injection that was intended to trigger only the lead follicle.
9.2 Methods of Stimulation
The difference between a successful stimulation and a defective stimulation leading to cycle cancellation has less to do with the choice of gonadotropin preparation and more to do with the administration protocol. The somewhat more simple monofollicular stimulation will be described here first, with the means for recruiting and sustaining the development of one or more additional follicles being reserved for the section on paucifollicular stimulation.
Basically, the parameters of “step-up,” “step-down,” or combined protocols are chosen to locate the FSH threshold, to manage the FSH window, and then to sustain appropriate follicular development so that only the optimal follicle continues to mature.
9.2.1 The Starting Dose
The prevailing consensus about an FSH threshold was first described 25 years ago by Brown and his colleagues [1], in an era prior to ultrasound technology. They also stressed the concept of inter-patient variability for this threshold, as well as more limited inter-cycle variations within the same patient. Because the ovarian follicle seems to respond to a particular level of FSH rather than to a particular administered dose, the issue is in fact how to characterize the relationship of injected dosage to resulting blood level. This is a complex multifactorial relationship that depends on severable variables:
Existing endogenous FSH secretion that occurs in every patient, except for those rare instances of hypogonadotropic hypogonadism
The route of administration, particularly when thick layers of subcutaneous fat create a “depot” effect at the injection site
The volume of distribution of injected FSH that is also somewhat related to the patient’s BMI
The metabolic clearance rate of the FSH preparation that varies according to its isoform composition and degree of sialylation, and also in relation to the patient’s age; these parameters all affect accumulation of hormone in plasma
Inter-patient variations of FSH threshold related to: (a) individual physiologic parameters specific to each patient, which can account for different FSH plasma levels resulting from identical injections, and (b) variable ovarian sensitivity to FSH within each patient, which explains why patients will react differently to the same FSH level
Relationships between plasma levels and FSH threshold have been extensively investigated by Schoemaker, who monitored plasma levels daily while making very precise adjustments to dosing in order to restrict variations in plasma on the order of 1 IU/l [2]. It was found that the quantity of injected gonadotropin necessary to obtain the same plasma FSH level could vary substantially from one patient to another, but that inter- and intra-patient variations in the ovarian response to a given FSH plasma level are much smaller, save for PCO patients. While “usual” patients can be expected to show no follicular response to blood levels below 7.8 IU/l, the actual FSH threshold can vary from 6.3 to 9.8 IU/l in PCO patients with large inter-individual variations being related to differences in ovarian sensitivity.
Aside from these variations between patients, the FSH threshold and thus the starting dose are also correlated to other parameters such as patient age, AFC, AMH level, and her BMI. Several attempts for predicting the best FSH starting dose have been proposed: for example, Frieseleben’s algorithm that takes into account only the patient’s AFC and weight [3]. However, these are only probabilities, and for an initial stimulation cycle it seems preferable to lean toward a lower dose if only because an insufficient dosage is easier to handle than an excessive one.
9.2.2 Selecting a Protocol
Schoemaker’s study also confirms that a minimal increase of FSH, as low as 1 IU/L above the threshold, may lead to multifollicular development. This finding illustrates two main basic principles:
The increment of administered gonadotropin doses must remain small, so that plasma FSH levels do not rise too rapidly. There is indeed a narrow range between the dose which elicits no response, even if administered indefinitely, and the dose responsible for multifollicular development. This dose range may not exceed 25–37.5 IU, and perhaps less.
The increment of FSH doses must remain progressive, as it takes 4–6 days for plasma hormone levels to stabilize, and also because the follicular recruitment period may spread over 5–15 days. This explains why FSH may be administered in three different types of protocol.
9.2.2.1 The “Step-Up” Protocols
Definitions of the protocols for gonadotropin administration began in the mid-1960s, when monitoring by measuring levels of urinary estrogens became available. The earlier stimulation method originally proposed by Crooke, that utilized very high single dosing or a fractionated 3-dose process was abandoned for one of two “step-up” designs [4]:
A Lunenfeld-Rabau proposal that each dose increment should be 75 IU (one HMG vial)
A proposal by Brown that increments should not increase over the previous dose by more than one-third
The Israeli protocol continues as the more frequently used, although many Australian clinicians have held strongly to Brown’s ideas.
9.2.3 The Standard Step Up Protocol
Basically because of its simplicity, this remains the most widely used protocol, particularly for spontaneously ovulating women. The fundamental approach begins with a daily FSH dose of 50–75 IU, with the first monitoring conducted after 5–7 days of administration. If no mature follicle is detected, there are three possible situations (Fig. 9.1):
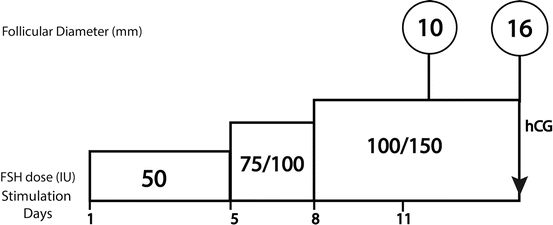

Fig. 9.1
Step-up standard protocol
A beginning follicular response, evidenced by the appearance at ultrasound of a follicle of at least 10 mm diameter plus a concomitant rise of plasma estradiol. In this case the stimulation should continue with the same FSH dose, and a second evaluation should be scheduled for 2–3 days later. Experience has shown that the starting dose can be continued throughout the complete stimulation cycle in most cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


