Definition and Classification
For the foregoing reasons, it seems logical that chronic hypertension would be defined as some level of sustained blood pressure that is associated with an increase in acute or long-term adverse effects. For many years in the United States, these values were based primarily on actuarial tables constructed using data derived from white adult males and compiled by life insurance companies. These “norms” disregarded interrelated factors such as ethnicity and gender as well as other important covariants. The importance of race, for example, was emphasized by Kotchen (2012), who cites statistics derived from 65 million American adults. In this study, the incidence of hypertension—defined as blood pressure > 140/90 mm Hg—was 34 percent in blacks, 29 percent in whites, and 21 percent in Mexican Americans.
For many years, guidelines for diagnosis, classification, and management of chronic hypertension have been promulgated by the Joint National Committee. In 2008, the National Heart Lung and Blood Institute discontinued these guidelines, and the Joint National Committee 8 (JNC 8) was instead asked to provide an evidence-based review (James, 2013). Findings pertinent to caring for young women with chronic hypertension are summarized in Table 50-1.
TABLE 50-1. Eighth Joint National Committee (JNC 8)—2014 Chronic Hypertension Guidelines and Recommendations
Evidence-based recommendations from randomized controlled trials
Definitions hypertension and prehypertension not addressed
Lifestyle modifications endorsed from the Lifestyle Work Group (Eckel, 2013)
Recommend selection among four specific medication classes: angiotensin-converting enzyme inhibitors (ACE-I), angiotensin-receptor blockers (ARB), calcium-channel blockers, or diuretics:
General population < 60 years old—initiate pharmacological therapy to lower diastolic pressure ≤ 90 mm Hg and systolic pressure ≤ 140 mm Hg
Diabetics—lower pressure < 140/90 mm Hg
Chronic kidney disease—lower pressure < 140/90 mm Hg. Also add ACE-I or ARB to improve outcomes
General nonblack population—initial therapy should include thiazide-type diuretic, calcium-channel blocker, ACE-I, or ARB
General black population—primary antihypertensive therapy should include thiazide-type diuretic or calcium-channel blocker
Assess monthly, and after 1 month, if goals not met, then increase primary drug dose or add second drug. If no response, increase either or add third drug; then if no response, refer to hypertension specialist
 Treatment and Benefits for Nonpregnant Adults
Treatment and Benefits for Nonpregnant Adults
There are proven benefits that accrue with treatment of otherwise normal adults who have sustained hypertension. A myriad studies evaluating many combinations of antihypertensive therapy have been conducted with salutary results. Some of these include the ACCORD, ASCOT, ACCOMPLISH, ALLHAT, SPRINT, TOHMS, TROPHY, and VALUE studies. Importantly, these trials evaluated monotherapy versus combination therapeutic regimens as well as ethnospecific benefits. Most evaluated cardiovascular outcomes, but many also confirmed risk reduction in cerebrovascular accidents, renal insufficiency, and overall mortality rates. Because of these incontrovertible benefits, the JNC 8 recommends the treatment outlined in Table 50-1.
Thus, even for mildly elevated blood pressures cited in Table 50-1, interventions to reduce pressure are beneficial. Moreover, it is clear that antihypertensive therapy in nonpregnant reproductive-aged women with sustained diastolic pressures ≥ 90 mm Hg would be considered standard. Not clear from these observations, however, is what constitutes the best management for the woman being treated who contemplates pregnancy, or the best management of the woman undergoing treatment who becomes pregnant, or the woman who is first identified to have chronic hypertension during pregnancy (August, 2014). In these and similar women, the benefits and safety of instituting antihypertensive therapy are less clear, as subsequently discussed.
 Preconceptional Counseling
Preconceptional Counseling
Women with chronic hypertension should ideally be counseled before pregnancy. The duration of hypertension, degree of blood-pressure control, and current therapy are ascertained. Home measurement devices should be checked for accuracy. Those who require multiple medications for control or those who are poorly controlled are also at increased risk for adverse pregnancy outcomes. General health, daily activities, and dietary habits are also assessed as shown in Table 50-2.
TABLE 50-2. Lifestyle Modifications for Hypertensive Patients—American Heart Association and American College of Cardiology
Consume a dietary pattern that emphasizes intake of vegetables, fruits, and whole grains; includes low-fat dairy products, poultry, fish, legumes, nontropical vegetable oils, and nuts; limits sweets and red meats—e.g. DASH dietary pattern, USDA Food Pattern, or the AHA Diet
Lower sodium intake—consume no more than 2400 mg sodium/day; 1500 mg/day desirable
Engage in aerobic physical activity three to four sessions per week, lasting on average 40 minutes per session, and involving moderate-to-vigorous intensity physical activity
In those with hypertension for more than 5 years or in diabetic women, cardiovascular and renal function should be assessed (August, 2014; Gainer, 2005). Women with evidence for organ dysfunction or those with prior adverse events such as cerebrovascular accident, arrhythmias, ventricular failure, or myocardial infarction are at markedly increased risk for a recurrence or worsening during pregnancy. Renal function is assessed by serum creatinine measurement, and proteinuria is quantified if the urine spot protein/creatinine ratio is abnormally high (Hladunewich, 2011). The Working Group Report on High Blood Pressure in Pregnancy (2000) of the National Heart, Lung, and Blood Institute concluded that the risks of fetal loss and accelerated deterioration of renal disease are increased if serum creatinine level is above 1.4 mg/dL (Chap. 53, p. 1061).
Although pregnancy is considered by many to be contraindicated in women with severe, poorly controlled hypertension, there is not a consensus regarding this. Certainly, pregnancy is at least relatively contraindicated in women who maintain persistent diastolic pressures of ≥ 110 mm Hg despite therapy, who require multiple antihypertensives, or who have a serum creatinine > 2 mg/dL. Even stronger contraindications include prior cerebrovascular accident, myocardial infarction, or cardiac failure.
DIAGNOSIS AND EVALUATION IN PREGNANCY
Classification of the hypertensive disorders complicating pregnancy is discussed in Chapter 40 (p. 728). Women are diagnosed with chronic hypertension if it is documented to precede pregnancy or if hypertension is identified before 20 weeks’ gestation. In some women without overt chronic hypertension, there is a history of repeated pregnancies complicated by gestational hypertension with or without the preeclampsia syndrome. Each is a risk marker for latent chronic hypertension, and this is especially so for preeclampsia, especially early-onset preeclampsia. In many ways, gestational hypertension is analogous to gestational diabetes in that such women have a chronic hypertensive diathesis, in which heredity plays a major role (Chap. 40, p. 769).
Although uncommon, secondary causes of hypertension are always a possibility in these women. Thus, consideration is given to an underlying pheochromocytoma, connective-tissue disease, Cushing syndrome, chronic renal disease, and myriad other causes. That said, most pregnant women with antecedent hypertension will have uncomplicated disease. As discussed above, some women—especially those with long-term or untreated hypertension—have complications that increase the risk of adverse pregnancy events. Thus, if not already accomplished, assessment during pregnancy is done for the cardiovascular system, the kidneys, and the cerebrovascular circulation.
 Associated Risk Factors
Associated Risk Factors
Several factors increase the likelihood that pregnant women will have chronic hypertension. Three of those most frequently cited are ethnicity, obesity, and diabetes. As previously discussed, chronic hypertension has a population incidence that is highest in black and lower in white and Mexican-American women (Kotchen, 2012). Related to this, hundreds of blood pressure-related phenotypes and genomic regions have been identified, including candidate genes for preeclampsia and chronic hypertension (Cowley, 2006; Lévesque, 2004).
The metabolic syndrome with superimposed preeclampsia is a risk marker for persistent hypertension postpartum (Spaan, 2012). This is not surprising because obesity may increase the prevalence of hypertension tenfold, and it is an important factor predisposing to chronic hypertension (Chap. 48, p. 963). In addition, obese women are more likely to develop superimposed preeclampsia. Diabetes is also prevalent in chronically hypertensive women, and its interplay with obesity and preeclampsia is overwhelming. In the study of more than 56 million births cited above, the most common comorbidities associated with chronic hypertension were pregestational diabetes—6.6 percent, thyroid disorders—4.1 percent, and collagen-vascular disease—0.6 percent (Bateman, 2012). Similar comorbidities were described by Cruz and associates (2011).
 Effects of Pregnancy on Chronic Hypertension
Effects of Pregnancy on Chronic Hypertension
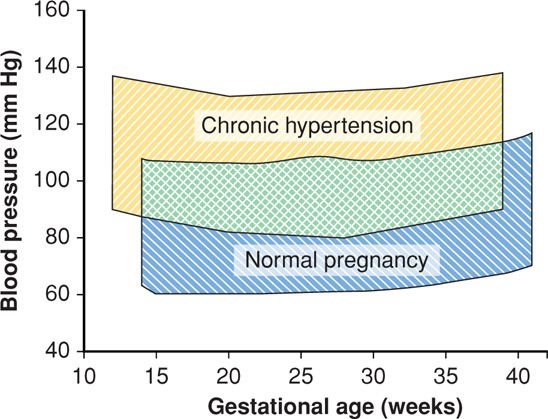
Blood pressure falls in early pregnancy in most women with chronic hypertension. It rises again during the third trimester (Fig. 50-1). According to studies by Tihtonen and coworkers (2007), women with chronic hypertension have persistently elevated vascular resistance and possibly a reduced intravascular volume increase. There is no doubt that adverse outcomes in these women are dependent largely on whether superimposed preeclampsia develops. This may be related to observations reported by Hibbard and colleagues (2005, 2014) that arterial mechanical properties are most marked in women with superimposed preeclampsia.
FIGURE 50-1 Mean systolic and diastolic blood pressures across pregnancy in 107 untreated chronically hypertensive women (yellow) compared with blood pressures across pregnancy in 4589 healthy nulliparous women (blue). (Data from August, 2014; Levine, 1997; Sibai, 1990a.)
ADVERSE PREGNANCY EFFECTS
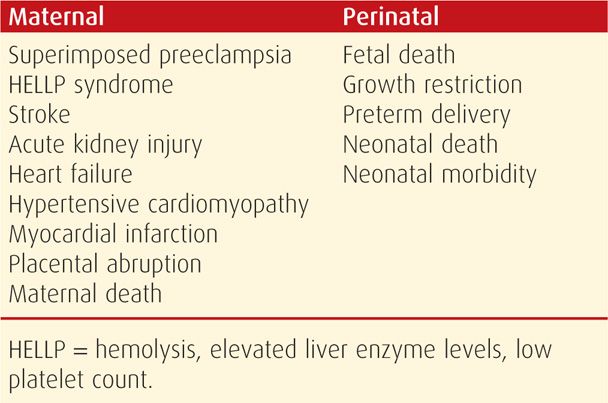
Chronic hypertension is associated with several adverse maternal and perinatal outcomes listed in Table 50-3. The recurring theme is that these are directly related to severity and duration of hypertension before pregnancy and whether superimposed preeclampsia develops, especially early in gestation. In women with mild chronic hypertension, outcomes are also related to blood pressure levels during pregnancy (Ankumah, 2013).
TABLE 50-3. Some Adverse Effects of Chronic Hypertension on Maternal and Perinatal Outcomes
 Maternal Morbidity and Mortality
Maternal Morbidity and Mortality
Most women whose hypertension is well controlled with monotherapy before pregnancy will do well. Even these women, however, are at increased risk for adverse outcomes. Complications are more likely with severe baseline hypertension and especially with documented end-organ damage (Czeizel, 2011; Odibo, 2013). In a study of pregnancy outcomes in nearly 30,000 chronically hypertensive women, Gilbert and associates (2007) reported markedly increased maternal morbidity including stroke, pulmonary edema, and renal failure. These observations were verified in the report from the Nationwide Patient Sample of more than 56 million deliveries by Bateman and colleagues (2012). Complications of hypertension included stroke—2.7 per 1000, acute renal failure—5.9 per 1000, pulmonary edema—1.5 per 1000, mechanical ventilation—3.8 per 1000, and in-house maternal mortality—0.4 per 1000. The contribution of hypertension to pregnancy-related strokes is discussed in Chapter 60 (p. 1191) and to hypertensive and idiopathic peripartum hypertensive cardiomyopathy in Chapter 49 (p. 988).
Pregnancy-aggravated hypertension may be due to gestational hypertension or to superimposed preeclampsia. In either instance, blood pressures can be dangerously elevated. As emphasized by Clark and Hankins (2012), systolic pressure ≥ 160 mm Hg or diastolic pressure ≥ 110 mm Hg will rapidly cause renal or cardiopulmonary dysfunction or cerebral hemorrhage. With superimposed severe preeclampsia or eclampsia, the maternal prognosis is poor unless the pregnancy is ended. Placental abruption is a common and serious complication (Chap. 41, p. 793). In addition to hypertensive heart failure mentioned above, aortic dissection has been described by Weissman-Brenner and coworkers (2004) and is discussed in Chapter 49 (p. 992).
Chronic hypertension has been associated with a fivefold risk for maternal death (Gilbert, 2007). This is emphasized by the report by Berg (2010) describing 4693 pregnancy-related deaths in the United States from 1998 through 2005. Hypertensive disorders, including chronic hypertension and preeclampsia syndrome, accounted for 12.3 percent of these deaths. Undoubtedly related were other causes of death such as cardiovascular conditions—12.4 percent, cerebrovascular conditions—6.3 percent, and cardiomyopathy—11.5 percent. Moodley (2007) reported similar findings with 3406 maternal deaths from South Africa. Interestingly, Sibai and associates (2011) reported that chronically hypertensive women with a history of preeclampsia were not at higher risk for complications compared with hypertensive women without such a history.
Superimposed Preeclampsia
Because there is no precise definition of superimposed preeclampsia in women with chronic hypertension, the reported incidence is variable. The risk is directly related to the severity of baseline hypertension. In a Maternal-Fetal Medicine Units Network trial, Caritis and coworkers (1998) identified superimposed preeclampsia in 25 percent. It was 29 percent in the California database study cited above (Yanit, 2012). According to the American College of Obstetricians and Gynecologists (2012), mild chronic hypertension has a 20-percent incidence for superimposed preeclampsia, whereas with severe hypertension, it is 50 percent. August and colleagues (2014) posit that this predilection may be because of similarities of genetic, biochemical, and metabolic abnormalities. In the study cited above, a history of superimposed preeclampsia did not increase the risk for recurrence, however, it was a marker for increased preterm delivery (Sibai, 2011).
Thus far, prognostic and predictive tests for superimposed preeclampsia have been disappointing when used clinically (American College of Obstetricians and Gynecologists, 2012; Conde-Agudelo, 2014; Zeeman, 2003). For example, Di Lorenzo and colleagues (2012) studied serum markers for Down syndrome and reported a sensitivity of 60 percent with a 20-percent false-positive rate. Similar results were found using antiangiogenic factors to discriminate among chronic hypertension, gestational hypertension, and preeclampsia (Sibai, 2008; Woolcock, 2008). According to Elovitz and coworkers (2013), microRNA assays may prove valuable as predictors of pregnancy-associated hypertension. This subject is discussed further in Chapter 40 (p. 747).
Prevention of Superimposed Preeclampsia
Trials of various substances to prevent preeclampsia in women with chronic hypertension have generally been disappointing. Low-dose aspirin has been evaluated most frequently. In the Network study by Caritis and associates (1998) cited above, the incidence of superimposed preeclampsia, fetal-growth restriction, or both was similar in women given low-dose aspirin or placebo. That said, Duley (2007) and Meads (2008) and their colleagues performed Cochrane reviews and found that low-dose aspirin was beneficial in some high-risk women. Minimal benefits were also found from a metaanalysis (Askie, 2007). To the contrary, however, the PARIS Collaborative Group (2007) reviewed individual data from 33,897 pregnant women enrolled in 33 randomized trials of low-dose aspirin and reported that women with chronic hypertension derived no benefits.
Spinnato and coworkers (2007) randomly assigned 311 women with chronic hypertension to antioxidant treatment with vitamins C and E or to a placebo. A similar number in both groups developed preeclampsia—17 versus 20 percent, respectively.
Placental Abruption
There seems to be no doubt that chronic hypertension increases the risk two- to threefold for premature placental separation (American College of Obstetricians and Gynecologists, 2012). As discussed in Chapter 41 (p. 793), the overall risk is one in 200 to 300 pregnancies, and this is increased to 1 in 60 to 120 pregnancies in women with chronic hypertension (Ananth, 2007; Cruz, 2011; Madi, 2012; Tuuli, 2011). This risk is increased further if the woman smokes or if she develops superimposed preeclampsia. The risk is highest with severe hypertension, and Vigil-De Gracia and colleagues (2004) reported it to be 8.4 percent. Most abruptions are in women with worsening gestational hypertension or superimposed preeclampsia. From the Norwegian Birth Registry, supplemental folic acid slightly decreased the abruption incidence in women with chronic hypertension (Nilsen, 2008).
 Perinatal Morbidity and Mortality
Perinatal Morbidity and Mortality
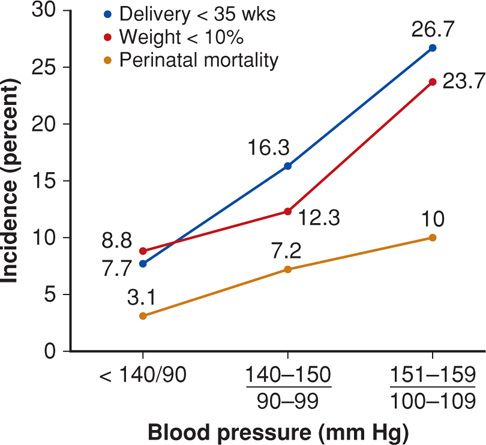
From the foregoing, it is not surprising that almost all adverse perinatal outcomes are increased in women with chronic hypertension. Some of those from a Network study are shown in Figure 50-2. The increasing incremental adverse effects of rising blood pressure levels are apparent. Interestingly, Bánhidy and colleagues (2011) have provided data suggesting that severe hypertension may be associated with fetal esophageal atresia or stenosis. As expected, for the entire group of women, those who developed preeclampsia had substantially increased adverse outcome rates compared with those without preeclampsia.
FIGURE 50-2 Perinatal outcomes in 759 women with midrange chronic hypertension enrolled in a Maternal-Fetal Medicine Units Network study (Ankumah, 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree