Study
Years
No. of patients
Regimen
Findings
1973–1978
342
VAC vs. VACD vs. VAC + Pulm XRT
Patients receiving DOX experienced best outcomes
Patients receiving DOX developed fewer metastases than those receiving XRT
Pelvic lesions have worse prognosis
IESS-II (Burgert et al. 1990)
1978–1982
214
VACD (DOX high-dose intermittent vs. moderate-dose continuous)
Improved disease control and survival with more aggressive chemotherapy
Early intensity improves outcomes
Increased cardiovascular toxicity with high-dose DOX
ET-1 (Craft et al. 1997)
1978–1986
142
VACD (± surgery)
Intensified neoadjuvant regimen improved resection of pelvic masses
Extremely poor outcomes for metastatic disease
Surgical outcomes better than radiation alone?
CESS 81 (Jürgens et al. 1988)
1981–1985
93
VACD
Decreased metastasis with early initiation of therapy
Increased local recurrence without complete resection despite radiation
CESS-86 (Paulussen et al. 2001)
1986–1991
301
Standard risk: VACD
High risk: VAD/IFOS
High-risk patients experienced same 10-year OS (52 % vs. 51 %)
Histologic response prognostic of outcome
ET-2 (Craft et al. 1998)
1987–1993
243
VCR + DOX + IFOS + DACT
CPM to IFOS and increased DOX improved outcomes
REN2 (Bacci et al. 1998)
1988–1991
82
VACD/IE
No benefit with adding IE relative to outcomes in REN1
INT-0091 (Grier et al. 2003)
1988–1992
518
VACD vs. VACD/IE
IE improved outcomes in nonmetastatic disease
No benefit to IE in metastatic disease
INT-0154 (Granowetter et al. 2009)
1995–1998
478
VDC + IE (standard dose vs. increased dose)
Increased dose therapy did not improve outcome
AEWS-0031 (Womer et al. 2012)
2001–2005
587
VDC + IE ± G-CSF (standard vs. interval compressed)
Interval compression improved outcome without increasing toxicity
6.2.4 Multi-agent Chemotherapy
With the identification throughout the late 1960s and early 1970s of multiple agents having activity against Ewing’s sarcoma, a number of small case series reported increasingly noteworthy successes utilizing multi-agent and multimodal therapies. A number of investigators reported responses, including durable remissions, in patients treated with VAC (vincristine, dactinomycin, cyclophosphamide) (Jaffe et al. 1976) and VACD (VAC plus doxorubicin) (Rosen et al. 1974). The report from Memorial Sloan Kettering was particularly impressive, describing 12 children treated with VACD, all of whom remained alive without disease for 10–37 months. Their report included three patients who initially presented with metastatic disease who also responded to therapy. These initial reports clearly opened the field for investigation into the appropriate and optimal application of adjuvant multi-agent and multimodal therapy. These studies also identified the management of toxicity as a primary obstacle to the widespread use of multi-agent chemotherapy, with high rates of congestive heart failure, infections complicating therapy, profound nausea and vomiting, as well as other side effects. Much of the research activity in subsequent years focused on managing these toxicities to make regimens more tolerable without sacrificing efficacy.
Establishment of the VACD backbone
In 1973, a collaborative effort between pediatric cancer groups was formed called the Intergroup Ewing’s Sarcoma Study. From 1973 to 1978, they performed their first clinical trial, which compared VAC to VACD and to VAC with prophylactic pulmonary radiotherapy (Nesbit et al. 1981). Analysis at 2.5 years found significant advantages to treatment with doxorubicin or pulmonary radiotherapy when compared to VAC alone (Nesbit et al. 1981). Repeat analysis after 6 years of follow-up further confirmed the benefits of adding doxorubicin to VAC therapy. In that analysis, patients receiving VACD experienced overall survival (OS) rates of 65 %, compared with only 28 % with VAC alone (Nesbit et al. 1990). While there was some survival advantage seen with the addition of pulmonary radiotherapy (OS of 53 %), the gains were much greater with doxorubicin, and the number of patients developing metastatic disease was much higher without it. Local recurrence rates remained unacceptably high—approximately 15 % across all groups. Subgroup analysis found that patients with pelvic disease fared much worse than patients with axial disease. At 5 years, OS was 34 % for pelvic disease versus 57 % in non-pelvic disease (Nesbit et al. 1990). This study, now known as IESS-1, firmly established the importance of doxorubicin in the treatment of patients with Ewing’s sarcoma. These results helped to confirm on a larger scale similar results reported by others (Rosen et al. 1978).
Building on the success of IESS-1, the intergroup subsequently set out to compare two competing regimens, one using intermittent high-dose therapy and another using continuous moderate-dose therapy. This second study, IESS-2, was performed between 1978 and 1982. Given the huge improvements in outcome seen in patients receiving doxorubicin, patients randomized to the high-dose therapy arm received a regimen similar to that used in IESS-1, only with increased doses of doxorubicin and cyclophosphamide. Five-year follow-up showed even greater gains in survival for patients receiving the intensified dose, with overall survival rates of 77 %, compared to 63 % in the moderate-dose therapy group (Burgert et al. 1990). Patients receiving the higher doses also experienced better disease control, with only 21 % of patients developing metastasis compared to 30 % of those in the moderate-dose therapy group. It was notable that increasing doses did not result in increased toxicities, with comparable adverse effects seen in both groups.
Trials conducted by multiple other cooperative groups confirmed the value of dose-intensive doxorubicin. Limiting their study to that of patients with localized bony disease, the group from St. Jude reported excellent results by adding doxorubicin both neoadjuvantly and adjuvantly in their ES-79 trial. Patients receiving doxorubicin had improved metastatic control, tumor regression, and overall survival. The five-year overall survival for these patients approached 80 % (Hayes et al. 1989). Results were similar in the collaborative POG 8346 study, where doxorubicin was administered in both the neoadjuvant and adjuvant settings. Response rate was found to be 88 % after neoadjuvant therapy (Donaldson et al. 1998). The 5-year OS of this group, which included those with pelvic and metastatic disease, was 55 %. Patients with localized disease had 5-year OS of 65 %.
The cooperative European group’s CESS-81 study likewise showed a 70 % response rate with 3-year DFS of responders at 79 % using a VACD-based regimen (Jürgens et al. 1988). Post hoc analysis performed in this study suggested that early intensification reduced rates of metastasis. Results from this study emphasized the need to improve local control. The establishment of centralized radiation planning mid-study reduced local recurrence rates of approximately 50 % early in the study to less than 25 % over the entire 6-year observation period (Jürgens et al. 1988). The ET-1 study, organized by investigators in the United Kingdom, again confirmed the need for intense early chemotherapy with VACD (Craft et al. 1997). This study further emphasized the need for aggressive local control, which is discussed in Chap. 9. The investigators made a point to emphasize the incredibly poor outcomes of patients with metastatic disease. While 45 of the 120 patients with localized disease were alive at 10 years, only 2 of the 20 patients presenting with metastatic disease survived 10 years beyond diagnosis, despite this aggressive chemotherapy regimen.
Management of pelvic disease
Given the poor outcomes noted in previous studies for patients with pelvic and sacral Ewing, the IESS-II study sought to improve outcomes in patients with pelvic disease. In this study, all patients with pelvic disease received intensified therapy with intermittent dosing using VACD. The need for appropriate local control was emphasized, and all patients with unresectable disease or with positive margins received intensive radiation with 55 Gy to the tumor bed with generous margins (Evans et al. 1991). Reported separately from the rest of the IESS-II trial, patients with pelvic disease receiving treatment under this intensified regimen experienced 5-year remission-free survival rates of 55 %, a dramatic improvement over the 23 % seen in the first IESS trial. These modifications in therapy, with intensification of chemotherapy and an emphasis on effective local control, pushed the 5-year OS in patients with pelvic disease very near than seen in patients with localized disease of the extremity. One should note, however, that multiple patients in this treatment cohort experienced late relapses at 6 years and later—a phenomenon particularly common to pelvic disease and a problem that continues to this day and that should be considered in the early reporting of trial results.
Addition of ifosfamide and etoposide
Seeking to capitalize on promising early reports of the activity of ifosfamide in Ewing’s sarcoma (discussed above), the two American groups (CCG and POG) conducted a collaborative study to investigate whether the addition of IE to standard therapy would improve outcome (Grier et al. 2003). INT-0091 demonstrated a survival benefit for patients with localized disease receiving courses of alternating VACD and IE. The addition of IE in this study increased the 5-year event-free survival (EFS) from 54 to 69 % in patients with localized disease. There was no benefit seen for patients with metastatic disease, with both the control and experimental groups experiencing a 22 % 5-year survival.
The group from the United Kingdom took a slightly different approach to the integration of ifosfamide in their ET-2 trial. Rather than alternating courses of VACD with IE, patients enrolled in ET-2 received cycles of ifosfamide, vincristine, and doxorubicin (IVAD) throughout both the neoadjuvant and adjuvant phases of therapy. In the last several cycles, dactinomycin replaced doxorubicin in order to limit cumulative doses of doxorubicin. The five-year OS improved from 44 % in ET-1 to 62 % in ET-2 (Craft et al. 1998). Importantly, the benefits of ifosfamide-based therapy also extended to patients who presented with metastatic disease. Five-year survival for this group increased from 9 to 23 %. Patients receiving ifosfamide in this study also experienced decreased incidence of local relapse. It is likely that this large benefit was more of a function of the markedly poor survival seen in the small number of patients with metastatic disease enrolled in ET-1 than it was a superiority of the IVAD regimen, as the two studies reported essentially identical results in patients with metastatic disease.
Dose intensification
Analyses performed in the early 1990s suggested that outcomes in patients with Ewing’s sarcoma depended largely on the dose intensity of chemotherapy, especially with regard to doxorubicin (Smith et al. 1991). From 1995 to 1998, the INT-0154 study sought to determine whether increasing the doses of medications in the already-established VDC + IE backbone could improve outcomes in patients with localized disease (Granowetter et al. 2009). Unfortunately, there were no benefits seen with intensification of therapy by this method, despite the increased numbers of toxicities observed. The five-year EFS was 70 % for the intensified regimen vs. 72 % for the standard regimen.
Interval compression
An alternate means of dose intensification was born with the development during the 1990s of marrow-stimulating agents such as filgrastim, which proved effective in accelerating marrow recovery. Using these medications, chemotherapy could be given more often. It was hypothesized that more frequent administration of cytotoxic agents would allow less time for recovery and expansion of chemotherapy-resistant clones within tumors. Preliminary studies testing this theory (Womer et al. 2000) suggested that “interval compression” facilitated by the routine use of colony-stimulating factors and less stringent count recovery criteria for the initiation of subsequent cycles could improve both EFS (73 %) and OS (85 %). Investigators designed the AEWS0031 study to determine the value of interval compression in a large-scale randomized setting. Patients enrolled in the study received the same chemotherapy dosage of VDC + IE either in traditional 3-week cycles or every 2 weeks by accelerating count recovery after chemotherapy with filgrastim (Womer et al. 2012). Interval compression improved both EFS and OS in patients with localized disease (from 65 to 73 % and 77 to 83 %, respectively). Importantly, interval compression did not increase toxicity. This regimen of interval compression with VDC + IE forms the backbone of current standard therapy regimens in North America and elsewhere.
6.2.5 Current Standard of Care
The treatment of patients with newly diagnosed Ewing’s sarcoma continues to build on a backbone of vincristine, doxorubicin, and cyclophosphamide. INT 0091 and ET-2 established a role for ifosfamide and etoposide as well. AEWS0031 demonstrated the value of interval compression. The most recent standard regimen, built on the successful evolution of these studies, is illustrated in Fig. 6.1. Treatment under this protocol consists of alternating cycles of VDC and IE, which repeat for a total of 14 cycles of therapy. Recovery is supported by the administration of G-CSF (granulocyte-colony stimulating factor) at the conclusion of each cycle. Subsequent cycles begin 14 days after the beginning of the last cycle or as soon as the patient demonstrates adequate hematologic recovery (defined by platelets greater than 75,000/μL and absolute neutrophil count (ANC) greater than 750/μL). Local control with surgery and/or radiation therapy is recommended after the sixth cycle of chemotherapy. If necessary, radiation can begin during cycle 7. Doxorubicin is omitted from the last two cycles of VDC to reduce cumulative anthracycline exposure.
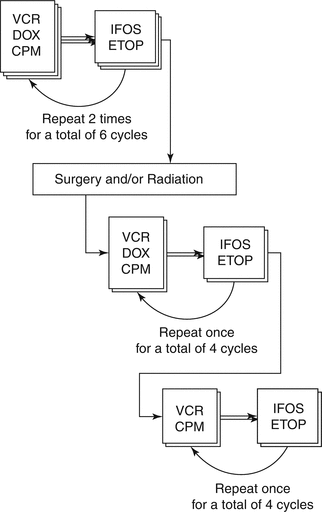

Fig. 6.1
Schematic overview of treatment recommendations for Ewing’s sarcoma. Based on the experimental arm used in AEWS0031, this interval compressed regimen is currently considered the standard of care in North America and elsewhere. See text for dosing details
Standard dosing for these agents is as follows:
VCR: vincristine 2 mg/m2 (max 2 mg), day 1
DOX: doxorubicin 37.5 mg/m2/day, days 1 and 2
CPM: cyclophosphamide 1,200 mg/m2, day 1 with MESNA for uroprotection
IFOS: ifosfamide 1,800 mg/m2/day, days 1–5 with MESNA for uroprotection
ETOP: etoposide 100 mg/m2/day IV infusion over 1–2 h, days 1–5
G-CSF: filgrastim 5 mcg/kg/day SQ, max 300 mcg, beginning 24–36 h after the last dose of chemotherapy, continued for at least 7 days or until the ANC is at least 750, or pegfilgrastim 100 mcg/kg SQ, max 6 mg, once 24–36 h after the last dose of chemotherapy
The failure of multiple attempts to improve outcomes by increasing doses of these agents above those used in typical modern regimens suggests that conventional doses of these agents probably lie somewhere near the ceiling of their therapeutic windows.
6.2.6 Ongoing and Upcoming Trials
A number of European centers favor induction using vincristine, ifosfamide, doxorubicin, and etoposide (VIDE, as used in the Euro-E.W.I.N.G. 99 study); this is considered to be an acceptable alternative standard of care. Euro-E.W.I.N.G 99 was designed to determine the safety and efficacy of this intensified induction regimen utilizing VIDE. It was also designed to compare the efficacy of consolidation using vincristine/dactinomycin/ifosfamide (VAI) with that of mega therapy consolidation using busulfan/melphalan in patients with isolated pulmonary metastases or poor responses to induction chemotherapy and to compare the tolerability and efficacy of consolidation therapy with VAI with that of consolidation with VAC in good responders. Investigators noted a lower-than-expected rate of poor response to induction therapy early in the study, which threatened accrual to the intermediate-risk arm. The study was opened to members of the Children’s Oncology Group under AEWS0331 in 2004 and was recently closed to accrual. Initial reports from this study have shown that the VIDE induction regimen can be given safely (Juergens et al. 2006) and that the prognostic value of different EWS fusion types may be questionable (Le Deley et al. 2010).
The successor to AEWS0031, AEWS1031, began recruiting in late 2010. This study aims to test the effects of incorporating topotecan, an inhibitor of topoisomerase, into the VDC + IE backbone, essentially alternating topotecan and doxorubicin in each subsequent cycle. The rationale for this study comes from activity observed for combined cyclophosphamide/topotecan regimens in patients with refractory/relapsed Ewing’s sarcoma (Hunold et al. 2004; Saylors et al. 2001) and from animal studies demonstrating more-than-additive activity when topotecan is combined with vincristine (Thompson et al. 1999). The study will continue to utilize interval compression supported by routine utilization of growth factor with less stringent count recovery requirements. Recruitment is ongoing. Results will not be known for several years to come.
6.3 Chemotherapy for Osteosarcoma
Prior to the advent of chemotherapy, only a small fraction of patients survived encounters with osteosarcoma. With the introduction of adjuvant chemotherapy in the 1960s and early 1970s, combined modality treatments improved the outlook. In little more than a decade, survival surged to over 60 %. Since the 1980s, however, improvements on these outcomes have proved elusive (Allison et al. 2012) despite myriad large-scale efforts testing promising therapies. Multiple agents that appeared promising in preclinical and early clinical studies proved disappointing in phase III trials. Efforts to intensify treatments have bought little change in survival, while efforts to reduce intensity and spare patients from many of the most harmful side effects had very negative impacts on outcomes. The search for more cures has been plagued by controversies concerning the very value of adjuvant chemotherapy and by study results that have proved difficult to interpret. There is a clear need for new therapies that can prevent and treat metastatic disease as current therapies remain ineffective in the treatment of disseminated osteosarcoma. Following is an overview summarizing approaches to the systemic treatment of osteosarcoma, including the early history of systemic therapy and identification of active agents, the approaches to therapy that have been tested in cooperative group trials, current standards of care, and emerging concepts that are being addressed in ongoing clinical trials.
6.3.1 Identification of Active Agents
Cyclophosphamide
Having observed the responses of patients with leukemia and various solid tumors, physicians started using cyclophosphamide to treat osteosarcoma during the early 1960s (Pinkel 1962; Finklestein et al. 1969; Sutow et al. 1971). While these early studies reported responses to cyclophosphamide in some patients, only about 15 % of patients showed objective responses. Treatment with higher doses or for longer periods of time was limited by a number of toxicities, especially hemorrhagic cystitis and persistent emesis. These limitations to use were eventually overcome with improvements in supportive care and preventive treatments, especially the advent of effective antiemetics (Cunningham et al. 1987) and of agents which can prevent urotoxicity (Scheef et al. 1979). Throughout the development of combination therapies, cyclophosphamide remained a staple of medical therapy, though it has since been largely abandoned, as its use has not enhanced outcomes beyond those obtained with current standard therapies.
Dactinomycin
In some of the first studies using animal models of osteogenic sarcoma, the RNA polymerase inhibitor dactinomycin showed significant activity against the Ridgway osteogenic sarcoma model (Schwartz et al. 1966, 1968). While concern over treatment-related toxicities prevented early adoption of dactinomycin for treating patients with osteosarcoma, some human studies were performed using the agent as a radiosensitizer that did show activity (Cupps et al. 1969). Interest in dactinomycin was renewed after VAC (vincristine, dactinomycin, and cyclophosphamide) showed efficacy in patients with rhabdomyosarcoma. Early studies utilizing VAC plus radiation therapy for patients with metastatic pulmonary disease achieved durable remissions in small numbers of patients (Sutow et al. 1975).
Methotrexate
Building on reports of the efficacy of antifolates in children with non-Hodgkin’s lymphoma and in adults with lung carcinomas, oncologists from the Children’s Hospital Boston group assessed the effects of high-dose methotrexate in patients with osteosarcoma. Their case series, published in 1973, showed the responses of ten patients with osteosarcoma (mostly metastatic) to an escalating dose regimen of high-dose methotrexate with citrovorum rescue (Jaffe et al. 1973). Nine of ten patients showed some response, including two patients who showed complete regression of lung metastases without radiation. By 1975, Rosen et al. showed improvement of 3-year EFS to 52 % by using high-dose methotrexate (HDMTX) with doxorubicin and cyclophosphamide, which together formed one of the first multi-agent protocols, deemed the T4 protocol (Delépine et al. 1990). Subsequent trials demonstrated a clear relationship to dose and response, which has driven multiple efforts to maximize methotrexate exposure (Winkler et al. 1984; Saeter et al. 1991). Methotrexate remains an integral component of standard therapy regimens to this day.
Doxorubicin
came of age during the early 1970s. Investigators from the Acute Leukemia Group assessed the efficacy of this agent in patients with osteosarcoma and showed that single-agent doxorubicin coupled with amputation increased disease-free survival to 45 % after 32 months (Cortes et al. 1974). Multiple subsequent studies showed marked efficacy of doxorubicin, including augmentation of response when added to other effective agents (Bacci et al. 1993; Smeland et al. 2003). The importance of doxorubicin for treating osteosarcoma was clarified by the COSS-82 study (Winkler et al. 1988). This study aimed to determine whether patients could be spared to highly toxic chemotherapy by withholding neoadjuvant doxorubicin (and cisplatin), supposing that poor histologic responders could be salvaged by introducing these agents to their adjuvant regimens. Results showed that patients who did not receive neoadjuvant doxorubicin fared poorly. The subsequent addition of doxorubicin to regimens of those who had poor histologic responses could not salvage their outcomes. This highlighted the importance of early dose intensity of doxorubicin in the treatment of osteosarcoma.
A meta-analysis performed on published studies in the late 1980s suggested that of all the agents employed in the treatment of osteosarcoma to date, doxorubicin had the greatest effect on outcome (Smith et al. 1991). The analysis also suggested that the dose intensity (amount of drug per unit time) had greater effect than total dose. Attempts were made early in the use of doxorubicin to push the dose of doxorubicin higher, though these efforts caused unacceptably high rates of cardiotoxicity (Von Hoff 1979). Analysis suggested that cumulative doses less than 240 mg/m2 were generally safe, but that rates of cardiotoxicity increased markedly with higher doses (Von Hoff 1979; Lipshultz et al. 1995). Data also suggested that decreasing the rate of administration could reduce the risk for developing cardiomyopathies (Lipshultz et al. 1995), but this has not played out in pediatric studies (Lipshultz et al. 2012). Doxorubicin continues to prove itself a very important drug in the treatment of osteosarcoma, with many believing it to be the most important single agent in modern regimens (Eselgrim et al. 2006).
The primary dose-limiting toxicity of doxorubicin has always been the development of cardiac failure and chemotherapy-related cardiotoxicity. Dexrazoxane has been developed as a cardioprotective agent. When given prior to chemotherapy, it can significantly reduce the rates of cardiotoxicity (Kalam and Marwick 2013) without reducing the therapeutic efficacy of doxorubicin. The use of dexrazoxane is recommended in patients receiving cumulative doses of anthracyclines that present a significant risk for substantial cardiac damage (van Dalen et al. 2005). Dexrazoxane continues to be increasingly utilized in protocols recommending higher doses of doxorubicin, which has become a routine (Anderson 2005; Mulrooney et al. 2009).
Cisplatin
A platinum-based DNA cross-linking agent, it was introduced in the late 1970s and quickly found favor with those treating osteosarcoma. In 1978, Ochs et al. reported responses in six of eight patients with disease recurrence after treatment with HDMTX and doxorubicin (Ochs et al. 1978). Several other studies have confirmed response rates in relapsed disease of approximately 40 % (Baum et al. 1979; Winkler et al. 1983). Ettinger et al. treated 12 newly diagnosed patients with cisplatin and doxorubicin in a neoadjuvant fashion and observed durable responses in most patients, with no evidence of disease in 10 out of 12 patients at a median follow-up of 23 months (Ettinger et al. 1981). In the COSS-82 study, the combination of doxorubicin/cisplatin was found to be far superior to BCD (bleomycin, cyclophosphamide, dactinomycin) (Winkler et al. 1988). While tolerated doses can be limited by nephrotoxicity and ototoxicity, cisplatin remains a primary agent for the treatment of osteosarcoma, a primary component of the MAP (methotrexate, adriamycin [doxorubicin], platinum [cisplatin]) backbone used in most modern regimens.
Ifosfamide (and etoposide)
Ifosfamide, another nitrogen mustard derivative, has been used as an alkylating agent in chemotherapy protocols since the early 1980s. A report by Marti et al. in 1985 sparked interest in ifosfamide as treatment for osteosarcoma. They reported clinical responses in one-third of patients with recurrent osteosarcoma (Marti et al. 1985). The COSS-86 study incorporated ifosfamide into the neoadjuvant treatment regimen and showed an improvement in histologic tumor response when added to the MAP backbone (Winkler et al. 1990). Investigators found evidence for synergy between ifosfamide and etoposide in recurrent sarcomas (Miser et al. 1987b), and data from the Rizzoli IOR-II study suggested that the combination may have improved outcomes for poor responders (Bacci et al. 1993). Based on these data, the Scandinavian group replaced the upfront regimen (MAP) with ifosfamide and etoposide (IE) alone as the salvage regimen for poor responders in the SSG-VIII study (Smeland et al. 2003). Unfortunately, their results showed that patients receiving IE alone as salvage therapy fared much worse than patients who continued to receive MAP. The INT-0133 study set out to definitively determine whether there was any benefit to augmenting the MAP backbone by adding ifosfamide. The results of that study were difficult to interpret (Meyers et al. 2005) due to an interaction in the statistical analysis of the factorial study design. In the most recent follow-up data (Meyers et al. 2008), however, it appears that the addition of ifosfamide by itself to the backbone regimen did not improve outcomes. Given these unclear results, many have continued to use ifosfamide or IE in the treatment of patients with osteosarcoma, though the data to support this has not been robust. The EURAMOS-1 study was designed to directly answer this question by comparing MAP-IE to MAP alone in a large cohort of poor histologic responders (Marina et al. 2014). The results of that study suggest that augmentation of standard MAP therapy with IE provides no clear survival benefit for patients with poor histologic response (see Chap. 8), while it definitively carries a cost of increased toxicity. Whether or not this more intensive regimen would be beneficial for patients with a good histologic response (i.e., those proved to be the most chemoresponsive) has not been tested.
Some suggest that ifosfamide may be more effective for osteosarcoma when given in doses much higher (15–18 mg/m2) than those typically used in most protocols (12 mg/m2). Whether high-dose ifosfamide truly provides additional benefit, and whether those benefits are warranted given the significant additional renal and hematologic toxicity, remains controversial. Trials directly comparing the efficacy of high-dose ifosfamide to standard-dose ifosfamide have produced conflicting results (Berrak et al. 2005; Ferrari et al. 2005; Kudawara et al. 2013). Some have expressed that the marked toxicities observed with these therapies do not justify even the best results noted in the previous studies. Regardless, many patients with recurrent, refractory, and metastatic disease have been treated with high-dose ifosfamide and etoposide, and this is generally considered an appropriate treatment.
6.3.2 Collaborative Groups
While osteosarcoma is the most common primary malignant tumor of the bone, it remains a relatively rare disease when compared to other malignancies and disease entities. The need to increase patient numbers in order to expedite and enhance the study of treatments for osteosarcoma was apparent early, and multiple collaborative groups formed across the United States and Europe. These have included the German-Austrian-Swiss study group, which has produced the COSS trials; the Scandinavian group, which has produced the SSG trials; the Italian group from the Rizzoli Institute; the European Osteosarcoma Intergroup (EOI) headquartered in Great Britain; and the North American groups, now consolidated under the Children’s Oncology Group, among others. More recent trials have been conducted under the umbrella of EURAMOS, which has organized all of these groups into one large international collaborative group for conducting clinical trials in patients with osteosarcoma.
A summary of the collaborative group studies undertaken to date is outlined in Table 6.2. Important findings from those studies and the ways in which they have influenced our current standard of care are given in the sections that follow.
Table 6.2
Osteosarcoma trials discussed in the text
Study | Years | No. of patients | Regimen | Findings |
|---|---|---|---|---|
T10 (Rosen et al. 1982) | 1978–1982 | 57 | Neo: HDMTX + DOX + BCD Adj: GR: HDMTX + DOX + BCD PR: DOX + CDDP + BCD | Suggested value to “salvage” therapy, showing equivalent responses in PRs switched from HDMTX to CDDP CDDP more effective than HDMTX? 93 % DFS at a median of 20 months from start of chemo |
COSS-80 (Winkler et al. 1983) | 1979–1982 | 192 | Neo: MTX + DOX + VCR + XRT Adj: MTX + DOX + VCR + BCD | No adverse outcomes from delaying definitive surgery No change in natural history of disease Definitive benefit of adjuvant chemotherapy |
COSS-82 (Winkler et al. 1988) | 1982–1984 | 141 | Neo: HDMTX + BCD vs. MAP Adj: GR: HDMTX + BCD or MAP PR: DOX + CDDP or CDDP + IFOS + BCD | BCD worse than DOX + CDDP Delayed administration of DOX led to poor outcomes Could not salvage poor responders by adding back DOX + CDDP Confirmed prognostic value of histologic necrosis |
SSG-II (Saeter et al. 1991) | 1982–1989 | 107 | Neo: HDMTX Adj: GR: HDMTX + DOX + BCD PR: HDMTX + DOX + CDDP + BCD | Single-agent MTX not effective as neoadjuvant therapy Confirmed dose-response for serum MTX and tumor necrosis Salvage chemotherapy was not effective |
CCG 782 (Provisor et al. 1997) | 1983–1986 | 268 | Neo: HDMTX + BCD Adj: GR: HDMTX + DOX + BCD PR: DOX + CDDP + BCD | Unable to confirm same outcomes as published for T10 protocol Validated histological response as prognostic tool Unable to salvage PR with CDDP |
EOI-1 (Bramwell et al. 1992) | 1983–1986 | 198 | DOX + CDDP vs. MAP | No improvement in outcome with addition of HDMTX Similar outcomes using simplified regimen |
IOR/OS-1 (Bacci et al. 1990) | 1983–1986 | 127 | Neo: MTX (HD vs. MD) + IA CDDP Adj: GR: MTX + CDDP FR: MTX + DOX + CDDP PR: DOX + BCD | Improved outcomes with HDMTX relative to standard dose Very poor outcomes in PR salvaged with DOX + BCD |
MIOS (Link et al. 1986) | 1986 | 36 | DOX + CDDP + BCD | Improved outcomes with adjuvant multi-agent chemotherapy |
1986–1988 | 171 | LR: MAP HR: MAP (IV vs. IA CDDP) + IFOS | Improved outcomes in patients receiving IFOS Increased histologic response with addition of IFOS No improved histologic response or survival benefit with IA CDDP | |
IOR/OS-2 (Bacci et al. 1993) | 1986–1989 | 164 | Neo: MAP (IA CDDP) Adj: GR: MAP PR: MAP + IE | PR salvaged with MAP + IE had outcomes similar to GR Increased heart failure with DOX doses >390 mg/m2 |
EOI-2 (Souhami et al. 1997) | 1986–1991 | 391 | DOX + CDDP vs. Neo: HDMTX + DOX + VCR Adj: HDMTX + DOX + CDDP + BCD | Equivalent outcomes with 2-drug regimen and T10 protocol Improved tolerability and decreased cost of simplified regimen |
T12 (Meyers et al. 1998) | 1986–1993 | 73 | Neo: HDMTX + BCD ± DOX + CDDP Adj: MAP + BCD | Intensified neoadjuvant regimen produced modest increase in histologic response but no improvement in EFS |
POG 8651 (Goorin et al. 2003) | 1986–1993 | 100 | MAP + BCD Surgery week 0 vs. week 10 | Delaying definitive surgical resection did not affect outcome |
SSG-VIII (Smeland et al. 2003) | 1990–1997 | 113 | Neo: HDMTX + DOX + CDDP Adj: GR: HDMTX + DOX + CDDP PR: IE | Improved outcomes when DOX and CDDP added to neoadjuvant therapy No improvement in outcome for PR by switching to IE response did not increase survival |
IOR/OS-3 (Ferrari et al. 1999) | 1993–1995 | 95 | Neo: MAP (IA vs. IV CDDP) Adj: GR: MAP PR: MAP + IFOS | Decreased cardiotoxicity with cumulative doses of DOX <390 mg/m2
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|