Fig. 12.1
A sagittal ultrasound image of a uterus with a cervical pregnancy
My Management
a.
Perform a suction curettage immediately upon presentation.
b.
Perform a bilateral uterine artery embolization (UAE) followed by a suction and curettage.
c.
Administer a single dose of methotrexate.
d.
Administer multidose methotrexate.
Diagnosis and Assessment
CEP is defined as a pregnancy that implants in the surface of the endocervical canal [1]. Its incidence is approximately 1 in 9000 pregnancies and accounts for 0.15 % of all ectopic pregnancies [2]. Our patient had several risk factors for cervical pregnancy including a history of a previous curettage, which is present in 50–70 % of patients with cervical pregnancy, and a history of a previous cesarean delivery, found in 16.7–37.5 % of patients with cervical pregnancy and pregnancy after IVF. Cervical pregnancy accounts for 0.1 % of all IVF pregnancies and 3.7 % of all IVF ectopic pregnancies. Other risk factors specific to cervical pregnancy include previous instrumentation or trauma to the cervix and Asherman’s syndrome [2–5].
Classically, cervical pregnancy presents as painless vaginal bleeding after a period of amenorrhea in 90.1 % of cases. On speculum examination, a disproportionately enlarged and softened cervix is seen [6]. Differential diagnosis include incomplete abortion, placenta previa, carcinoma of the cervix, and degenerating leiomyoma [6].
The first ultrasound of cervical pregnancy was reported by Raskin in 1978 [7]. Almost a decade later, Ushakov described the ultrasound criteria of cervical pregnancy . They arep: gestational sac in the endocervix, intact cervical canal above the gestational sac, closed internal cervical os, trophoblastic invasion of the endocervix by Doppler ultrasound, the presence of embryo/fetal structures/cardiac activity, empty uterine cavity, endometrial decidualization, and hourglass uterus [2]. Cervical pregnancy can be differentiated from an aborting intrauterine pregnancy that is stuck in the cervical canal by a “sliding sac sign.” The sac that slides with slight pressure of the endovaginal probe to the cervix suggests no intimate attachment between the gestational sac and the endocervical tissue. When available, an MRI can be used to confirm the diagnosis (Fig. 12.2).
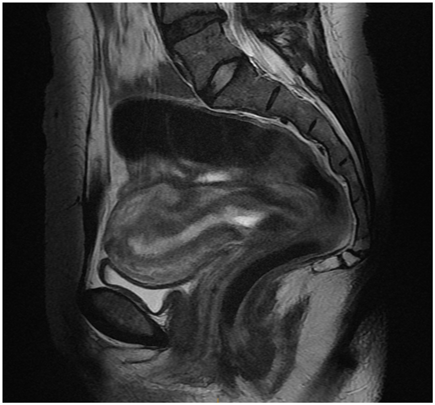

Fig. 12.2
A sagittal magnetic resonance imaging of the pelvis with a cervical pregnancy
In the past, CEPs were diagnosed late and many women ended up with hysterectomies as a consequence of catastrophic bleeding. Increased awareness and ultrasound examination provide means for early diagnosis and lead to decreased morbidity and mortality.
Management
Following the establishment of a diagnosis of cervical pregnancy, treatment should be immediately started.
Medical Management
In stable patients, medical treatment with methotrexate is preferable. Most of the data on cervical pregnancy are derived from case series, and most experts advocate treatment with multidose methotrexate regimen. Due to the high risk of severe bleeding from cervical pregnancy leading to hysterectomy, we advise against single-dose methotrexate regimen and local injection of methotrexate for the treatment of CEP. We recommend administering methotrexate 1 mg/kg body weight intramuscularly on days 1, 3, 5, and 7 alternating with leucovorin 0.1 mg/kg body weight intramuscularly on day 2, 4, 6, and 8. In general, the effectiveness of methotrexate alone to treat CEP is reduced when serum β-hCG level > 10,000 IU/L, gestational age > 9 weeks, presence of fetal cardiac activity, and crown-rump length (CRL) > 10 mm [8].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


