John C. Elkas
Cervical cancer ranks as the third most common gynecologic neoplasm in the United States, behind cancer of the corpus and ovary, mainly as a result of the effectiveness of screening programs. Worldwide, cervical carcinoma continues to be a significant health care problem. In developing countries, where health care resources are limited, cervical carcinoma is the second most frequent cause of cancer death in women. Because cervical cancer is preventable, it is imperative that gynecologists and other primary health care providers to women be familiar with vaccination programs, screening techniques, diagnostic procedures, and risk factors for cervical cancer and management of preinvasive disease. Vaginal cancer is a rare tumor that shares an epidemiology and risk factor profile that is similar to cervical cancer.
Cervical Cancer
Epidemiology and Risk Factors
Invasive cancer of the cervix is considered a preventable disease because it has a long preinvasive state, cervical cytology screening programs are currently available, and the treatment of preinvasive lesions is effective. In spite of the preventable nature of this disease, 12,710 new cases of invasive cervical cancer resulting in 4290 deaths were anticipated in the United States in 2011 (1). Nationally, the lifetime probability of developing cervical cancer is 1:128. Although screening programs in the United States are well established, it is estimated that 30% of cervical cancer cases will occur in women who have never had a Papanicolaou (Pap) test. In developing countries, this percentage approaches 60% (2). Nevertheless, the worldwide incidence of invasive disease is decreasing, and cervical cancer is being diagnosed earlier, leading to better survival rates (1,3). The mean age for cervical cancer in the United States is 47 years, and the distribution of cases is bimodal, with peaks at 35 to 39 years and 60 to 64 years of age (1).
There are numerous risk factors for cervical cancer: young age at first intercourse (younger than 16 years), multiple sexual partners, cigarette smoking, race, high parity, low socioeconomic status, and chronic immune suppression. The relationship to oral contraceptive use was debated. Some investigators proposed that use of oral contraceptives might increase the incidence of cervical glandular abnormalities; however, this hypothesis was not consistently supported (4,5). Many of these risk factors are linked to sexual activity and exposure to sexually transmitted diseases. Infection with the herpes virus was thought to be the initiating event in cervical cancer; however, infection with human papillomavirus (HPV) was determined to be the causal agent in the development of cervical cancer, with herpes virus and Chlamydia trachomatis likely acting as cofactors. The role of human immunodeficiency virus (HIV) in cervical cancer is mediated through immune suppression (4). The Centers for Disease Control and Prevention described cervical cancer as an acquired immune deficiency syndrome (AIDS)–defining illness in patients infected with HIV (6).
The initiating event in cervical dysplasia and carcinogenesis is infection with HPV. HPV infection was detected in up to 99% of women with squamous cervical carcinoma. HPV is the causative agent in both squamous and adenocarcinoma of the cervix, but the respective tumors may have different carcinogenic pathways (7). There are more than 100 different types of HPV, more than 30 of which can affect the lower genital tract. There are 14 high-risk HPV subtypes; two of the high-risk subtypes, 16 and 18, are found in up to 62% of cervical carcinomas. The mechanism by which HPV affects cellular growth and differentiation is through the interaction of viral E6 and E7 proteins with tumor suppressor genes p53 and Rb, respectively. Inhibition of p53 prevents cell cycle arrest and cellular apoptosis, which normally occurs when damaged DNA is present, whereas inhibition of Rb disrupts transcription factor E2F, resulting in unregulated cellular proliferation (8). Both steps are essential for the malignant transformation of cervical epithelial cells. Two HPV vaccines, the quadrivalent Gardasil and the bivalent Cervarix, are approved by the U.S. Food and Drug Administration (FDA) and protect against subtypes 16 and 18. After 3 years, the efficacy of Gardasil was 99% for preventing cervical intraepithelial neoplasia grades 2 and 3 caused by HPV 16 or 18 in females who were not previously infected with either HPV 16 or 18 before vaccination; however, efficacy was only 44% in those who were infected prior to vaccination (9). Because the quadrivalent and bivalent HPV vaccines both protect only against certain types of HPV, vaccinated women need to continue to receive Pap test screening according to guidelines.
Evaluation
Vaginal bleeding is the most common symptom occurring in patients with cancer of the cervix. Most often, this is postcoital bleeding, but it may occur as irregular or postmenopausal bleeding. Patients with advanced disease may present with a malodorous vaginal discharge, weight loss, or obstructive uropathy. In asymptomatic women, cervical cancer is most commonly identified through evaluation of abnormal cytologic screening tests. The false-negative rate for Pap tests in the presence of invasive cancer is up to 50%, so a negative Pap test should never be relied on in a symptomatic patient (10).
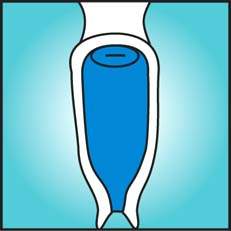
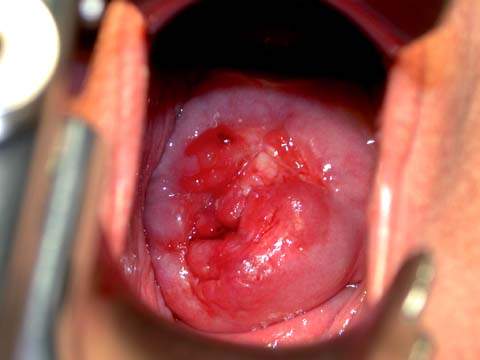
Initially, all women suspected of having cervical cancer should have a general physical examination performed to include evaluation of the supraclavicular, axillary, and inguinofemoral lymph nodes to exclude the presence of metastatic disease. On pelvic examination, a speculum is inserted into the vagina, and the cervix is inspected for suspicious areas (Fig. 36.1). The vaginal fornices also should be closely inspected. With invasive cancer, the cervix is usually firm and expanded, and these features should be confirmed by digital examination. Rectal examination is important to help establish cervical consistency and size, particularly in patients with endocervical carcinomas. Rectal examination is the only way to determine cervical size if the vaginal fornices have been obliterated by menopausal changes or by the extension of disease. Parametrial extension of disease is best determined by the finding of nodularity beyond the cervix on rectal examination.
Figure 36.1 Gross appearance of cervical cancer on examination.
When obvious tumor growth is present, a cervical biopsy is usually sufficient for diagnosis. If gross disease is not present, a colposcopic examination with cervical biopsies and endocervical curettage is warranted. If the diagnosis cannot be established conclusively with colposcopy and directed biopsies, which may be the case with adenocarcinoma, cervical conization may be necessary.
Colposcopic Findings of Invasion
Colposcopic examination is mandatory for patients with suspected early invasive cancer based on cervical cytology and a grossly normal-appearing cervix. Colposcopic findings that suggest invasion are (i) abnormal blood vessels, (ii) irregular surface contour with loss of surface epithelium, and (iii) color tone change. Colposcopically directed biopsies may permit the diagnosis of frank invasion and thus avoid the need for diagnostic cone biopsy, allowing treatment to be administered without delay. If there is debate about the depth of invasion based on the cervical biopsies, and if the clinical stage may be upstaged to stage IA2 or IB1, the patient should undergo a conization. In the presence of a large cervical biopsy specimen showing invasion greater than 3 mm, or two biopsy specimens separated by 7 mm showing invasive cervical carcinoma, therapy should proceed without delay, and the patient could undergo radical surgery or radiation therapy.
Abnormal Blood Vessels
Abnormal vessels may be looped, branched, or reticular. Abnormal looped vessels are the most common colposcopic finding and arise from the punctated and mosaic vessels present in cervical intraepithelial neoplasia (CIN). As the neoplastic growth process proceeds and the need for oxygen and nutrition increases, angiogenesis occurs as a result of tumor and local tissue production of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and other cytokines, resulting in the proliferation of blood vessels and neovascularization. Punctate vessels push out over the surface of the epithelium in an erratic fashion, producing the looped, corkscrew, or J-shaped pattern of abnormal vessels characteristic of invasive disease. Abnormal blood vessels arise from the cervical stroma and are pushed to the surface as the underlying cancer invades. The normally branching cervical stromal vessels are best observed over nabothian cysts. In this area, the branches are generally at acute angles, with the caliber of vessels becoming smaller after branching, much like the arborization of a tree. The abnormal branching blood vessels seen with cancer tend to form obtuse or right angles, with the caliber sometimes enlarging after branching. Sharp turns, dilations, and luminal narrowing also characterize these vessels. The surface epithelium may be lost in these areas, leading to irregular surface contour and friability.
Abnormal reticular vessels represent the terminal capillaries of the cervical epithelium. Normal capillaries are best seen in postmenopausal women with atrophic epithelium. When cancer involves this epithelium, the surface is eroded, and the capillary network is exposed. These vessels are very fine and short and appear as small comma-shaped vessels without an organized pattern. They are not specific to invasive cancer; atrophic cervicitis may also have this appearance.
Irregular Surface Contour
Abnormal surface patterns are observed as tumor growth proceeds. The surface epithelium ulcerates as the cells lose intercellular cohesiveness secondary to loss of desmosomes. Irregular contour may occur as a result of papillary characteristics of the lesion. This finding can be confused with a benign HPV papillary growth on the cervix. For that reason, biopsies should be performed on all papillary cervical growths to avoid missing invasive disease.
Color Tone
Color tone may change as a result of increasing vascularity, surface epithelial necrosis, and in some cases, production of keratin. The color tone is yellow-orange rather than the expected pink of intact squamous epithelium or the red of the endocervical epithelium.
Adenocarcinoma
Adenocarcinoma of the cervix does not have a specific colposcopic appearance. All of the aforementioned blood vessels may be seen in these lesions. Because adenocarcinomas tend to develop within the endocervix, endocervical curettage is required as part of the colposcopic examination, and traditional screening methods are less reliable (10).
Histologic Appearance of Invasion
Cervical conization is required to assess correctly the depth and the linear extent of involvement when microinvasion is suspected. Early invasion is characterized by a protrusion of malignant cells from the stromal–epithelial junction. This focus consists of cells that appear better differentiated than the adjacent noninvasive cells and have abundant pink-staining cytoplasm, hyperchromatic nuclei, and small- to medium-sized nucleoli (11). These early invasive lesions form tonguelike processes without measurable volume and are classified as International Federation of Gynecology and Obstetrics (FIGO) stage IA1. With further progression, more tonguelike processes and isolated malignant cells appear in the stroma, followed by a proliferation of fibroblasts (desmoplasia) and a bandlike infiltration of chronic inflammatory cells (Fig. 36.2). With increasing depth of invasion, lesions occur at multiple sites, and the growth becomes measurable by depth and linear extent. Lesions that are less than 3 mm in depth are classified as FIGO stage IA1. Lesions that are 3 to 5 mm or more in depth and up to 7 mm in linear extent are classified as FIGO stage IA2 (12). As the depth of stromal invasion increases, so does the risk of capillary lymphatic space involvement. Dilated capillaries, lymphatic spaces, and foreign-body multinucleated giant cells containing keratin debris are often seen in the stroma.
Figure 36.2 Microinvasive squamous carcinoma. Multiple irregular tonguelike processes and isolated nests of malignant cells are seen, some surrounded by clear spaces, simulating capillary lymphatic invasion. This is an artifact caused by tissue shrinkage. The depth of stromal invasion is measured from the basement membrane of the overlying cervical intraepithelial neoplasia (CIN). In this case, it is 1.2 mm.
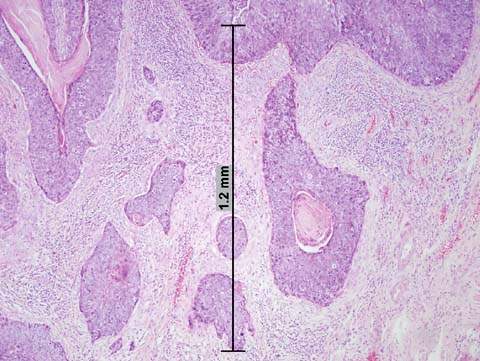
The depth of invasion should be measured with a micrometer from the base of the epithelium to the deepest point of invasion. Depth of invasion is a significant predictor for the development of pelvic lymph node metastasis and tumor recurrence. Although lesions that have invaded 3 mm or less rarely metastasize, patients in whom lesions invade between 3 to 5 mm have positive pelvic lymph nodes in 3% to 8% of cases (13). The significance of the cutoff at 3 mm is not identified completely; it was postulated that small capillary–lymphatic spaces at this level are incapable of facilitating the transport of malignant cells. Uneven shrinkage of tissue by fixative often creates space between the tumor nests and the surrounding fibrous stroma, simulating vascular lymphatic invasion (Fig. 36.2). Therefore, suspected vascular–lymphatic involvement with invasion of less than 3 mm should be interpreted with care. A lack of endothelial lining indicates that the space is a fixation artifact rather than true vascular invasion.
Staging
Cervical cancer is a clinically staged disease. The FIGO staging system is the standard and is applicable to all histologic types of cervical cancer. The FIGO staging system is presented in Table 36.1 and Figure 36.3. The staging procedures allowed by FIGO are listed in Table 36.2. When there is doubt concerning the stage to which a cancer should be allocated, the earlier stage should be selected. After a clinical stage is assigned and treatment is initiated, the stage must not be changed because of subsequent findings by either extended clinical staging or surgical staging. The upstaging of patients during treatment will produce an erroneous perception of improvement in the results of treatment of low-stage disease. Following is a breakdown of the incidence of cervical cancer by stage at diagnosis: 38%, stage I; 32%, stage II; 26%, stage III; and 4%, stage IV (3,13,14).
Additional Staging Modalities
Various investigators used lymphangiography, computed tomography (CT), ultrasonography, magnetic resonance imaging (MRI), and positron emission tomography (PET) in an attempt to improve the accuracy of clinical staging (15–25). These modalities suffer from poor sensitivity and high false-negative rates. Evaluation of the para-aortic lymph nodes with lymphangiography is associated with a false-positive rate of 20% to 40% and a false-negative rate of 10% to 20% (15–17). Overall, lymphangiography has a sensitivity of 79% and specificity of 73% (20). CT has poor sensitivity (34%) but excellent specificity (97%) (21). The accuracy of CT scanning is 80% to 85%; the false-negative rate is 10% to 15%, and the false-positive rate is 20% to 25% (16–18). Ultrasound has a high false-negative rate (30%), low sensitivity (19%), but high specificity (99%) (19). Early data showed that MRI results were comparable to those of CT scanning, a finding confirmed on meta-analysis (21,22). However, a systematic review comparing CT scan with MRI showed that MRI is significantly more sensitive with equivalent specificity. Additionally, MRI has excellent sensitivity on T2-weighted images for the detection of parametrial disease (23). As a result, MRI is the preferred study to evaluate tumor size, lymph node metastasis, and local tumor extension.
Table 36.1 FIGO Staging of Carcinoma of the Cervix Uteri (2008)
| Stage I | The carcinoma is strictly confined to the cervix (extension to the corpus would be disregarded) |
| IA | Invasive carcinoma which can be diagnosed only by microscopy, with deepest invasion ≤5 mm and largest extension ≤7 mm |
| IA1 | Measured stromal invasion of ≤3.0 mm in depth and extension of ≤7.0 mm |
| IA2 | Measured stromal invasion of >3.0 mm and not >5.0 mm with an extension of not >7.0 mm |
| IB | Clinically visible lesions limited to the cervix uteri or pre-clinical cancers greater than stage IAa |
| IB1 | Clinically visible lesion ≤4.0 cm in greatest dimension |
| IB2 | Clinically visible lesion >4.0 cm in greatest dimension |
| Stage II | Cervical carcinoma invades beyond the uterus, but not to the pelvic wall or to the lower third of the vagina |
| IIA | Without parametrial invasion |
| IIA1 | Clinically visible lesion ≤4.0 cm in greatest dimension |
| IIA2 | Clinically visible lesion >4 cm in greatest dimension |
| IIB | With obvious parametrial invasion |
| Stage III | The tumor extends to the pelvic wall and/or involves lower third of the vagina and/or causes hydronephrosis or non-functioning kidneyb |
| IIIA | Tumor involves lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydronephrosis or non-functioning kidney |
| Stage IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to Stage IV |
| IVA | Spread of the growth to adjacent organs |
| IVB | Spread to distant organs |
FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int Cynecol Obst 2009;105:103–104. aAll macroscopically visible lesions, even those with superficial invasion, are allotted to stage IB carcinomas. Invasion is limited to a measured stroma invasion with a maximal depth of 5.0 mm and a horizontal extension greater than 7.0 mm. Dept of invasion should not be greater than 5 mm taken from the base of the epithelium of the original tissue squamous or glandular. The depth of invasion should always be reported in millimeters, even those cases with “early minimal stromal invasion” (∼1 mm). The involvement of vascular/lymphatic spaces should not change stage allotment. bOn rectal examination, there is no cancer-free space between the tumor and the pelvic wall. All cases with hydronephrosis or non-functioning kidney are included, unless they are known to be due to another cause. | |
Figure 36.3 Carcinoma of the cervix uteri: staging cervical cancer (primary tumor and metastases). (From Benedet JL, Odicino F, Maisonneuve P, et al. Carcinoma of the cervix. J Epidemiol Biostat 2001;6:5–44, with permission.)
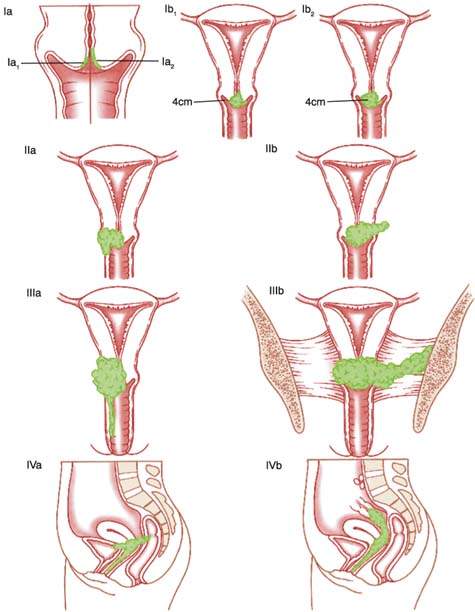
Table 36.2 Staging Procedures
| Physical examinationa | Palpate lymph nodes |
| Examine vagina | |
| Bimanual rectovaginal examination (under anesthesia | |
| recommended) | |
| Radiologic studiesa | Intravenous pyelogram |
| Barium enema | |
| Chest x-ray | |
| Skeletal x-ray | |
| Proceduresa | Biopsy |
| Conization | |
| Hysteroscopy | |
| Colposcopy | |
| Endocervical curettage | |
| Cystoscopy | |
| Proctoscopy | |
| Optional studiesb | Computerized axial tomography |
| Lymphangiography | |
| Ultrasonography | |
| Magnetic resonance imaging | |
| Positron emission tomography | |
| Radionucleotide scanning | |
| Laparoscopy | |
aAllowed by the International Federation of Gynecology and Obstetrics (FIGO). bInformation that is not allowed by FIGO to change the clinical stage. | |
PET scans are increasingly being utilized either alone or in conjunction with CT or MRI to detect metastatic disease; however, large prospective data series are limited. Early studies suggest that PET may be more useful than other techniques for the detection of abdominal and extrapelvic disease, with comparable or better sensitivity (76% to 100%) and specificity (94%) (24,25). In addition, PET scans may be better predictors of treatment outcome. Although early studies show promise for the use of PET scans in evaluating cervical cancer, the sensitivity for detecting metastatic disease less than 1 cm in size appears to be limited (26).
When abnormalities are noted on CT, MRI, or PET, radiographic-guided fine-needle aspirations (FNA) can be performed to confirm metastatic disease and individualize treatment planning. Because these tests are not available equally throughout the world and the interpretation of results can be variable, these studies are not used for staging. They may be useful in individual treatment planning.
The clinical staging system developed by FIGO is based on the belief that cervical cancer is a local disease until rather late in its course. The accuracy of clinical staging is limited, and surgical evaluation, although not practical or feasible in all patients, can more accurately identify metastatic disease. Surgical staging is advocated by providers who believe that surgical information details the extent of disease, allowing the treatment to be tailored to the individual (27). However, other providers believe that surgical staging should be limited to patients who are enrolled in clinical trials. These beliefs are based on the lack of randomized controlled studies demonstrating a survival benefit in patients who had surgical staging.
Pathology
Squamous Cell Carcinoma
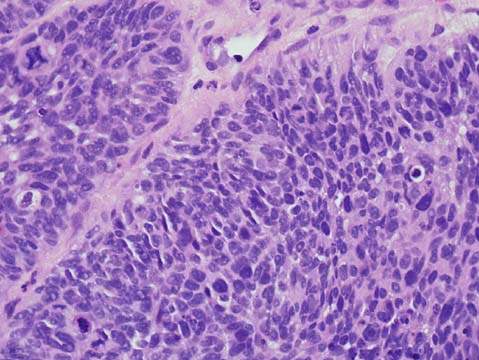
Invasive squamous cell carcinoma is the most common variety of invasive cancer in the cervix. Histologically, variants of squamous cell carcinoma include large cell keratinizing, large cell nonkeratinizing, and small cell types (28). Large cell keratinizing tumors consist of tumor cells forming irregular infiltrative nests with laminated keratin pearls in the center. Large cell nonkeratinizing carcinomas reveal individual cell keratinization but do not form keratin pearls (Fig. 36.4). The category of small cell carcinoma includes poorly differentiated squamous cell carcinoma and small cell anaplastic carcinoma. If possible, these two tumors should be differentiated. The former contains cells that have small- to medium-sized nuclei and more abundant cytoplasm than those of the latter. The designation of small cell anaplastic carcinoma should be reserved for lesions resembling oat cell carcinoma of the lung. Small cell anaplastic carcinoma infiltrates diffusely and consists of tumor cells that have scanty cytoplasm, round to oval small nuclei, coarsely granular chromatin, and high mitotic activity. The nucleoli are absent or small. Immunohistochemistry or electron microscopy can differentiate the small cell neuroendocrine tumors. Patients with the large cell type of carcinoma, with or without keratinization, have a better prognosis than those with the small cell variant. Small cell anaplastic carcinomas behave more aggressively than poorly differentiated squamous carcinomas that contain small cells. Infiltration of parametrial tissue and pelvic lymph node metastasis affect the prognosis.
Figure 36.4 Invasive squamous cell carcinoma, large cell nonkeratinizing type. Tumor cells form irregular nests and have abundant eosinophilic cytoplasm and distinct cell borders indicative of squamous differentiation.
Other less common variants of squamous carcinoma include verrucous carcinoma and papillary (transitional) carcinoma. Verrucous carcinomas may resemble giant condyloma acuminatum, are locally invasive, and rarely metastasize. Papillary carcinomas histologically resemble transitional cells of the bladder and may have more typical squamous cell invasion at the base of the lesion. Papillary carcinomas behave and are treated in a manner similar to traditional squamous cell cancers, except that late recurrences were noted.
Adenocarcinoma
There is an increasing number of cervical adenocarcinomas reported in women in their 20s and 30s. Although the total number of cases of adenocarcinoma is relatively stable, this disease is appearing more frequently in young women, especially as the number of cases of invasive squamous cell carcinoma decreases. Older reports indicated that 5% of all cervical cancers were adenocarcinomas, whereas newer reports show a proportion as high as 18.5% to 27% (29–31). Much of this proportional increase is related to a decreasing incidence of squamous carcinoma secondary to screening programs (which are less accurate at identifying preinvasion adenocarcinoma), greater exposure to oral contraceptives, and a greater exposure to HPV (4,5).
Adenocarcinoma in situ (AIS) is believed to be the precursor of invasive adenocarcinoma, and it is not surprising that the two often coexist (32). In addition to AIS, intraepithelial or invasive squamous neoplasia occurs in 30% to 50% of cervical adenocarcinomas (33). A squamous intraepithelial lesion may be observed colposcopically on the ectocervix, and the coexistent adenocarcinoma often is higher in the cervical canal.
Patients with AIS who are treated with conization should undergo close clinical follow-up. Endocervical curettage, often used in surveillance, may miss residual or invasive disease, and false-negative rates as high as 50% were reported (34). In addition, skip lesions not resected at the time of conization may be present. For these reasons, hysterectomy should be considered the standard therapy for patients who have completed their childbearing. In two reports, patients with negative cone biopsy margins were followed conservatively, with few requiring repeat surgical procedures (35,36). Because cervical AIS tends to affect women during their reproductive years, a thorough discussion of risks and benefits should take place, and treatment should be individualized.
Adenocarcinoma of the cervix is managed in the same manner used for squamous cell carcinoma. Adenocarcinoma was believed to be associated with a worse prognosis and outcome when compared with squamous cell carcinoma. A study of 203 women with adenocarcinoma and 756 women with squamous carcinoma supported this assertion (30). This study showed 5-year survival rates of 90% versus 60%, 62% versus 47%, and 36% versus 8% for stages I, II, and III, respectively. Although some attributed these rates to a relative resistance to radiation, they are more likely a reflection of the tendency of adenocarcinomas to grow endophytically and to be undetected until a large volume of tumor is present. When adjusted for tumor size, it appears that there is no difference in prognosis between the two histologic subtypes. Adenocarcinoma may be detected by cervical sampling, but less reliably so than squamous carcinomas. A definitive diagnosis may require cervical conization.
The clinical features of stage I adenocarcinomas are well studied (30,37–39). These studies identified size of tumor, depth of invasion, grade of tumor, and age of the patient as significant correlates of lymph node metastasis and survival. When matched with squamous carcinomas for lesion size, age, and depth of invasion, the incidence of lymph node metastases and the survival rate appear to be the same (38,39). Patients with stage I adenocarcinomas can be selected for treatment according to the same criteria as for those with squamous cancers (39).
The choice of treatment for bulky stage I and II tumors is controversial. Some advocated treatment with radiation alone, whereas others support radiation plus extrafascial hysterectomy (40–42). In 1975, Rutledge et al. reported an 85.2% 5-year survival rate for all patients with stage I disease treated with radiation alone and an 83.8% survival rate for those who had radiation plus surgery (41). The central persistent disease rate was 8.3%, compared with 4% for those who had radiation plus surgery. In stage II disease, the 5-year survival rate was 41.9% for radiation alone and 53.7% for radiation plus surgery. A subsequent report revealed no significant difference in survival among patients treated with radiation alone or radiation plus extrafascial hysterectomy (43).
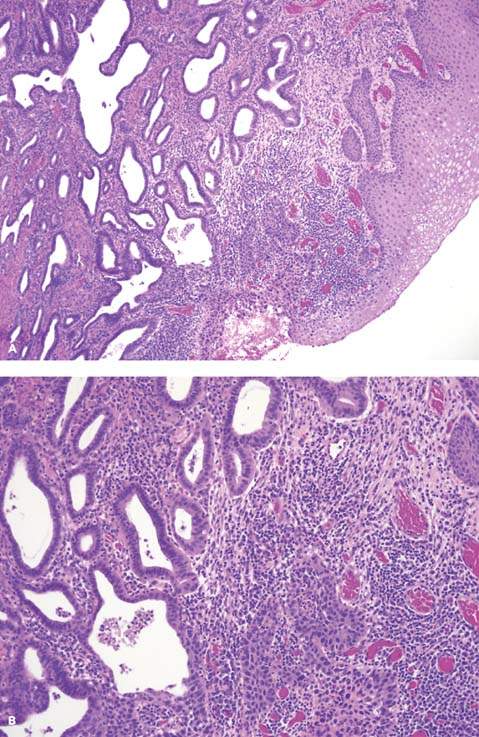
Invasive adenocarcinoma may be pure (Fig. 36.5A, B) or mixed with squamous cell carcinoma. Within the category of pure adenocarcinoma, the tumors are quite heterogeneous, with a wide range of cell types, growth patterns, and differentiation (30). About 80% of cervical adenocarcinomas consist predominantly of the endocervical type cells with mucin production. The remaining tumors are populated by endometrioid cells, clear cells, intestinal cells, or a mixture of more than one cell type. By histologic examination alone, some of these tumors are indistinguishable from those arising elsewhere in the endometrium or ovary. Within each cell type, the growth patterns and nuclear abnormalities vary according to the degree of differentiation. In well-differentiated tumors, tall columnar cells line the well-formed branching glands and papillary structures, whereas pleomorphic cells tend to form irregular nests and solid sheets in poorly differentiated neoplasms. The latter may require mucicarmine and periodic acid–Schiff (PAS) staining to confirm their glandular differentiation.
Figure 36.5 Invasive adenocarcinoma of the cervix, well-differentiated. A. Irregular glands are lined with tall columnar cells with vacuolated mucinous cytoplasm resembling endocervical cells. B. Nuclear stratification, mild nuclear atypism, and mitotic figures are evident in higher power.
There are several special variants of adenocarcinoma. Minimal deviation adenocarcinoma (adenoma malignum) is an extremely well-differentiated form of adenocarcinoma in which the branching glandular pattern strongly simulates that of the normal endocervical glands. The lining cells have abundant mucinous cytoplasm and uniform nuclei (44,45). Because of this, the tumor may not be recognized as malignant in small biopsy specimens, thereby causing considerable delay in diagnosis. Special immunohistochemical staining may be required to establish the diagnosis. Earlier studies reported a dismal outcome for women with this tumor, but more recent studies found a favorable prognosis if the disease is detected early (46). Although rare, similar tumors were reported in association with endometrioid, clear, and mesonephric cell types (47).
An entity described as villoglandular papillary adenocarcinoma deserves special attention (48). It primarily affects young women, some of whom are pregnant or users of oral contraceptives. Histologically, the tumors have smooth, well-defined borders, are well differentiated, and are either in situ or superficially invasive. Follow-up information is encouraging. None of these tumors recurred after cervical conization or hysterectomy, and no metastasis was detected among women undergoing pelvic lymphadenectomy. This tumor appears to have limited risk for spread beyond the uterus.
Adenosquamous Carcinoma
Carcinomas with a mixture of malignant glandular and squamous components are known as adenosquamous carcinomas. Patients with adenosquamous carcinoma of the cervix were reported to have a poorer prognosis than those with pure adenocarcinoma or squamous carcinoma (49). Whether this is true when corrected for size of lesion is controversial (38,39).
In mature adenosquamous carcinomas, the glandular and squamous carcinomas are readily identified on routine histologic evaluation and do not cause diagnostic problems. In poorly differentiated or immature adenosquamous carcinomas, however, glandular differentiation can be appreciated only with special stains, such as mucicarmine and PAS. In one study, 30% of squamous cell carcinomas demonstrated mucin secretion when stained with mucicarmine (47). These squamous cell carcinomas with mucin secretion have a higher incidence of pelvic lymph node metastases than do squamous cell carcinomas without mucin secretion, and they are similar to the signet-ring variant of adenosquamous carcinoma (47,50).
Glassy cell carcinoma is recognized as a poorly differentiated form of adenosquamous carcinoma (51). Individual cells have abundant eosinophilic, granular, ground-glass cytoplasm, large round to oval nuclei, and prominent nucleoli. The stroma is infiltrated by numerous lymphocytes, plasma cells, and eosinophils. Approximately half of these tumors contain glandular structures or stain positive for mucin. The poor diagnosis of this tumor is linked to understaging and resistance to radiotherapy.
Other variants of adenosquamous carcinoma include adenoid basal carcinoma and adenoid cystic carcinoma. Adenoid basal carcinoma simulates the basal cell carcinoma of the skin (51). Nests of basaloid cells extend from the surface epithelium deep into the underlying tissue. Cells at the periphery of tumor nests form a distinct parallel nuclear arrangement, so-called peripheral palisading. An “adenoid” pattern occasionally develops, with “hollowed-out” nests of cells. Mitoses are rare, and the tumor often extends deep into the cervical stroma.
Adenoid cystic carcinoma of the cervix behaves much like such lesions elsewhere in the body. The tumors tend to invade into the adjacent tissues and metastasize late, often 8 to 10 years after the primary tumor was removed. Like other adenoid cystic tumors, they may metastasize directly to the lung. The pattern simulates that of the adenoid basal tumor, but there is a cystic component, and the glands of the cervix are involved (51). Mitoses may be seen but are not numerous.
Sarcoma
The most important sarcoma of the cervix is embryonal rhabdomyosarcoma, which occurs in children and young adults. The tumor has grapelike polypoid nodules, known as botryoid sarcoma, and the diagnosis depends on the recognition of rhabdomyoblasts. Leiomyosarcomas and mixed mesodermal tumors involving the cervix may be primary but are more likely to be secondary to uterine tumors. Cervical adenosarcoma is described as a low-grade tumor with a good prognosis (52). If recurrence develops, it is generally a central recurrence that may be treated with resection and hormonal therapy.
Malignant Melanoma
On rare occasions, melanosis is seen in the cervix. Malignant melanoma may arise de novo in this area. Histopathologically, it simulates melanoma elsewhere, and the prognosis depends on the depth of invasion into the cervical stroma.
Neuroendocrine Carcinoma
The classification of neuroendocrine cervical carcinoma includes four histologic subtypes: (i) small cell, (ii) large cell, (iii) classical carcinoid, and (iv) atypical carcinoid (53). Neuroendocrine tumors of the cervix are rare, and treatment regimens are based on small case series of patients.
Small cell (neuroendocrine type) carcinoma of the cervix is aggressive in nature and is similar to cancer arising from the bronchus (54). The hallmark of neuroendocrine tumors is their aggressive malignant behavior with the propensity to metastasize. At the time of diagnosis, it is usually disseminated, with bone, brain, liver, and bone marrow being the most common sites of metastases. In one study of 11 patients with disease apparently confined to the cervix, a high rate of lymph node metastasis was noted (55). Pathologically, the diagnosis is aided by the finding of neuroendocrine granules on electron microscopy and by immunoperoxidase studies that are positive for a variety of neuroendocrine proteins such as calcitonin, insulin, glucagon, somatostatin, gastrin, and adrenocorticotropic hormone (ACTH). In addition to the traditional staging for cancer of the cervix, these patients should undergo bone, liver and brain scanning and bone marrow aspiration and biopsy to evaluate the possibility of metastatic disease. Therapyconsists of surgery, chemotherapy, and radiation. Because patients with early-stage disease have distant metastases, multimodal therapy is recommended. The main active chemotherapeutic agent is etoposide.
Local therapy alone gives almost no chance of cure of small cell carcinoma. Regimens of combination chemotherapy improved the median survival rates in small cell bronchogenic carcinoma, and these regimens are used for treatment of small cell carcinoma of the cervix. Combination chemotherapy may consist of vincristine, doxorubicin, and cyclophosphamide (VAC) or VP-16 (etoposide) and cisplatin (EP) (56). Patients must be monitored carefully because they are at high risk for developing recurrent metastatic disease (57).
Patterns of Spread
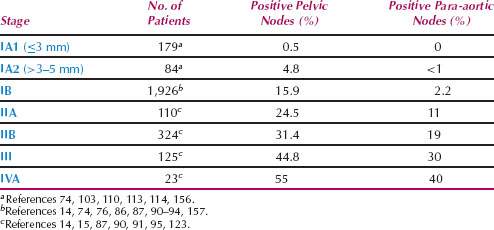
Cancer of the cervix spreads by (i) direct invasion into the cervical stroma, corpus, vagina, and parametrium; (ii) lymphatic metastasis; (iii) blood-borne metastasis; and (iv) intraperitoneal implantation. The incidence of pelvic and para-aortic nodal metastasis is shown in Table 36.3.
Table 36.3 Incidence of Pelvic and Para-aortic Lymph Node Metastasis by Stage
The cervix is commonly involved in cancer of the endometrium and vagina. The latter is rare, and most lesions that involve the cervix and vagina are designated cervical primaries. Consequently, the clinical classification is that of cervical neoplasia extending to the vagina, rather than vice versa. Endometrial cancer may extend into the cervix by three modes: direct extension from the endometrium, submucosal involvement by lymph vascular extension, and multifocal disease. The latter is most unusual, but occasionally a focus of adenocarcinoma may be seen in the cervix, separate from the endometrium. This lesion should not be diagnosed as metastasis but rather as multifocal disease. Malignancies involving the peritoneal cavity (e.g., ovarian cancer) may be found in the cul-de-sac and extend directly into the vagina and cervix. Carcinomas of the urinary bladder and colon occasionally extend into the cervix. Cervical involvement by lymphoma, leukemia, and carcinoma of the breast, stomach, and kidney is usually part of the systemic pattern of spread for these malignancies. Isolated metastasis to the cervix in such cases may be the first sign of a primary tumor elsewhere in the body.
Treatment Options
The treatment of cervical cancer is similar to the treatment of any other type of malignancy in that both the primary lesion and potential sites of spread should be evaluated and treated. The therapeutic modalities for achieving this goal include primary treatment with surgery, radiotherapy, chemotherapy, or chemoradiation. Whereas radiation therapy can be used in all stages of disease, surgery is limited to patients with stage I to IIa disease. The 5-year survival rate for stage I cancer of the cervix is approximately 85% with either radiation therapy or radical hysterectomy. A study using the National Cancer Institute’s Surveillance Epidemiology and End Results data by an intent-to-treat analysis showed that patients in the surgery arm had an improved survival when compared with patients in the radiation arm (58). Optimal therapy consists of radiation, or surgery alone, to limit the increased morbidity that occurs when the two treatment modalities are combined. Recent improvements in the treatment of cervical carcinoma include adjuvant chemoradiation in patients discovered to have high-risk cervical carcinoma after radical hysterectomy and in patients with locally advanced cervical carcinoma.
Surgery
There are advantages to the use of surgery instead of radiotherapy, particularly in younger women for whom conservation of the ovaries is important. Chronic bladder and bowel problems that require medical or surgical intervention occur in up to 8% of patients undergoing radiation therapy (59). Such problems are difficult to treat because they result from fibrosis and decreased vascularity. This is in contrast to surgical injuries, which usually can be repaired without long-term complications. Sexual dysfunction is less likely to occur after surgical therapy than radiation, because of vaginal shortening, fibrosis, and atrophy of the epithelium associated with radiation. Surgical therapy shortens the vagina, but gradual lengthening can be brought about by sexual activity. The epithelium does not become atrophic because it responds either to endogenous estrogen or to exogenous estrogens if the patient is postmenopausal.
Radical hysterectomy is reserved for women who are in good physical condition. Advanced chronologic age should not be a deterrent. With improvements in anesthesia, elderly patients withstand radical surgery almost as well as their younger counterparts (60). It is prudent not to operate on lesions that are larger than 4 cm in diameter because these patients will require postoperative radiation therapy. When selected in this manner, the urinary fistula rate is less than 2%, and the operative mortality rate is less than 1% (61,62). A summary of the management of cervical cancer is presented in Table 36.4.
Table 36.4 Management of Invasive Cancer of the Cervix
| Stage IA1 | ≤3 mm invasion, no LVSI | Conization or type I hysterectomy |
| ≤3 mm invasion, w/LVSI | Radical trachelectomy or type II radical hysterectomy with pelvic lymphadenectomy | |
| IA2 | >3–5 mm invasion | Radical trachelectomy or type II radical hysterectomy with pelvic lymphadenectomy |
| IB1 | >5 mm invasion, <2 cm | Radical trachelectomy or type III radical hysterectomy with pelvic lymphadenectomy |
| >5 mm invasion, >2 cm | Type III radical hysterectomy with pelvic lymphadenectomy | |
| IB2 | Type III radical hysterectomy with pelvic and para-aortic lymphadenectomy or primary chemoradiation | |
| Stage IIA1, IIA2 | Type III radical hysterectomy with pelvic and para-aortic lymphadenectomy or primary chemoradiation | |
| IIB, IIIA, IIIB | Primary chemoradiation | |
| Stage IVA | Primary chemoradiation or primary exenteration | |
| IVB | Primary chemotherapy ± radiation | |
| LVSI, lymphovascular space invasion. | ||
If radiation therapy is needed, transposing the ovaries out of the planned radiation field may preserve ovarian function. Although transposition provides some protection, studies suggest that normal ovarian function is preserved in fewer than 50% of patients (63,64). Metastasis to the ovaries occurs in 0.9% of cases of early stage cervical cancer, so preservation of the ovaries, particularly with adenocarcinoma, may confer a small recurrence risk (65).
Cone Biopsy of the Cervix
Cone biopsy of the cervix serves both a diagnostic and therapeutic role in cervical cancer. The procedure is indicated to confirm the diagnosis of cancer, and to definitively treat stage Ia1 disease when preservation of fertility is desired. For effective treatment, there must be no evidence of lymph–vascular space invasion, and both endocervical margins and curettage findings must be negative for cancer or dysplasia. Because stage Ia1 cancers have less than a 1% risk of lymph node metastasis, lymphadenectomy is not necessary. If the endocervical margin or curettage is positive for dysplasia or malignancy, further treatment is necessary because these findings are strong predictors of residual disease. For squamous cell carcinoma, the risk of residual disease is 4% if both the endocervical margin and curettage are negative for dysplasia or malignancy, 22% if the endocervical margin alone is positive, and 33% if both are positive (66). In cases of AIS, the status of the cone margins is particularly important, with residual preinvasive and invasive disease noted in up to 25% and 3%, respectively, of cases with negative margins, and up to 80% and 7%, respectively, in cases with positive margins (67,68).
Figure 36.6 Abdominal radical trachelectomy.
Simple (Extrafascial) Hysterectomy
Type I hysterectomy is an appropriate therapy for patients with stage Ia1 tumors without lymph–vascular space invasion who are not desirous of future fertility. In such cases, lymphadenectomy is not recommended. If lymph–vascular space invasion is found, a modified radical hysterectomy with pelvic lymphadenectomy is appropriate and effective therapy.
Radical Trachelectomy
Radical trachelectomy is a procedure that is gaining popularity as a surgical management option for women with stage 1A2 and IB1 disease who desire uterine preservation and fertility. This procedure may be performed vaginally, abdominally, laparoscopically, or robotically (Fig. 36.6), and it usually is accompanied by pelvic lymphadenectomy and cervical cerclage placement. The risk of positive pelvic lymph nodes with stage Ia2 cancer may be as high as 8%, indicating the need for lymphadenectomy. Lymphadenectomy may be performed laparoscopically, robotically, or by the open laparotomy technique. Experience with this therapeutic modality is limited, although early results are promising, and it is uncertain whether the long-term outcome is similar to that of traditional therapy. Patients who are ideal candidates for this procedure have tumors less than 2 cm in diameter and have negative lymph nodes. Lymphadenectomy can be performed at the beginning of the procedure, and depending on those results, the procedure can be continued or abandoned. A retrospective trial comparing patients who had tumors with these attributes and were treated with either laparoscopic radical hysterectomy or laparoscopic radical trachelectomy showed similar outcomes and recurrence (69). There are limited data on subsequent pregnancy outcomes after radical trachelectomy; however, successful outcomes were reported. A study found that for women attempting to conceive after radical trachelectomy, the 5-year cumulative pregnancy rate was 52.8%, with an increased risk of miscarriage (70). Although radical trachelectomy and lymphadenectomy are performed with curative intent, it should be remembered that if a recurrence develops, definitive therapy with surgery or radiation is necessary.
Radical Hysterectomy
The radical hysterectomy (Fig. 36.7A, B) performed most often in the United States is that described by Meigs in 1944 (71). The operation includes pelvic lymphadenectomy along with removal of most of the uterosacral and cardinal ligaments and the upper one-third of the vagina. This operation is referred to as the type III radical hysterectomy (72).
The hysterectomy described by Wertheim is less extensive than a radical hysterectomy and removes the medial half of the cardinal and uterosacral ligaments (62). This procedure is often referred to as the modified radical or type II hysterectomy. Wertheim’s original operation did not include pelvic lymphadenectomy but instead included selective removal of enlarged lymph nodes. The modified radical hysterectomy (type II) differs from the radical hysterectomy (type III) in the following ways:
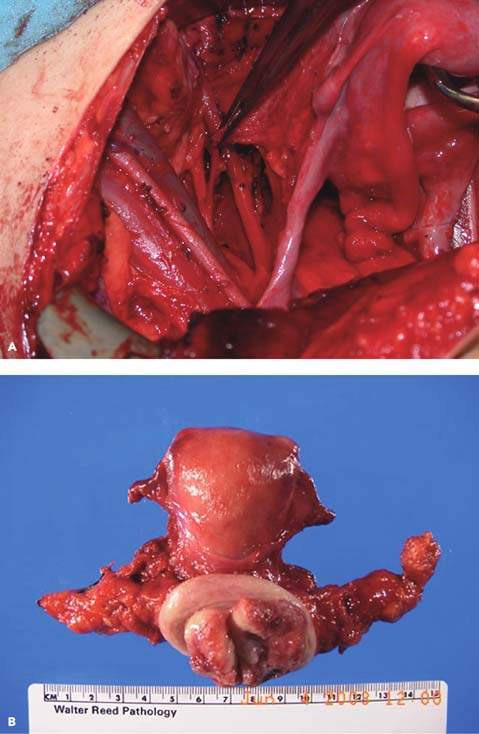
Figure 36.7 A: Radical hysterectomy. An intraoperative photograph showing the lateral dissection during a radical hysterectomy. Note the ureter running beneath the uterine artery (tissue in the clamp). B: Radical hysterectomy specimen.
Radical hysterectomies can be further classified as extended radical hysterectomy (type IV and type V). In the type IV operation, the periureteral tissue, superior vesicle artery, and as much as three-fourths of the vagina are removed. In the type V operation, portions of the distal ureter and bladder are resected. This procedure is rarely performed because radiotherapy should be used when such extensive disease is encountered (72).
The abdomen is opened through either a midline incision or a low transverse incision after the methods of Maylard or Cherney. The low transverse incision requires division of the rectus muscles and provides excellent exposure of the lateral pelvis. It allows adequate pelvic lymphadenectomy and wide resection of the primary tumor. After the abdomen is entered, the peritoneal cavity is explored to exclude metastatic disease. The stomach is palpated to ensure that it has been decompressed to facilitate packing of the intestines. The liver is palpated, and the omentum is inspected for metastases. Both kidneys are palpated to ensure their proper placement and lack of congenital and other abnormalities. The para-aortic nodes are palpated transperitoneally.
During exploration of the pelvis, the fallopian tubes and ovaries are inspected for any abnormalities. In premenopausal patients, the ovaries can be conserved. The peritoneum of the vesicouterine fold and the rectouterine pouch should be inspected for signs of tumor extension or implantation. The cervix is palpated between the thumb anteriorly and the fingers posteriorly to determine its extent, and the cardinal ligaments are palpated for evidence of lateral tumor extension or nodularity.
Lymphadenectomy
After inspection of the abdomen and pelvis, the pelvic and para-aortic lymph nodes should be inspected and palpated. Lymph nodes suspicious for gross disease should be excised and evaluated by frozen section. If metastatic disease is identified, consideration should be given to abandoning radical surgery in favor of primary chemoradiation therapy. If the patient has no gross evidence of metastatic disease, the pelvic lymphadenectomy is begun.
Pelvic Lymphadenectomy
The pelvic lymphadenectomy is begun by opening the round ligaments at the pelvic sidewall and developing the paravesical and pararectal spaces. The ureter is elevated on the medial flap by a Deaver retractor to expose the common iliac artery. The common iliac and external iliac nodes are dissected, with care taken to avoid injuring the genitofemoral nerve, which lies laterally on the psoas muscle. At the bifurcation of the common iliac artery, the external iliac node chain is divided into lateral and medial portions.
The lateral chain is stripped free from the artery to the circumflex iliac vein distally. A hemoclip is placed across the distal portion of the lymph node chain to reduce the incidence of lymphocyst formation. The medial chain is dissected. The obturator lymph nodes are dissected; for this procedure, the lymph nodes are grasped just under the external iliac vein, and traction is applied medially. In most patients the obturator artery and vein are dorsal to the obturator nerve; however, 10% have an aberrant vein arising from the external iliac vein. The node chain is separated from the nerve and vessels and clipped caudally. Dissection continues cephalad to the hypogastric artery. The cephalad portion of the obturator space should be entered lateral to the external iliac artery and medial to the psoas muscle, where the remainder of the obturator node tissue can be dissected as far cephalad as the common iliac artery. Drainage of the pelvic and para-aortic lymph node beds is not performed because of the increase in complications in patients in whom drains were used (73).
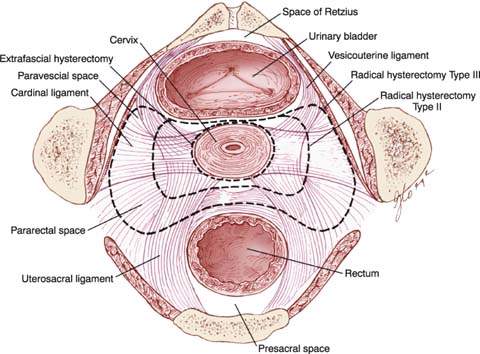
Figure 36.8 The pelvic ligaments and spaces. (From Berek JS, Hacker NF. Berek & Hacker’s Gynecologic Oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2010:360, with permission.)
Patients who have bulky cervical tumors or grossly positive pelvic nodes, or for whom frozen section evaluation will be performed, should undergo para-aortic lymph node evaluation to determine the full extent of disease and to guide adjuvant therapy.
Para-aortic Lymph Node Evaluation
The bowel is packed to expose the peritoneum overlying the bifurcation of the aorta. The peritoneum is incised medial to the ureter and over the right common iliac artery. A retractor is placed retroperitoneally to expose the aorta and the vena cava. Any enlarged para-aortic lymph nodes are removed, hemoclips are applied for hemostasis, and specimens are sent for analysis by frozen section. If the lymph nodes are positive for metastatic cancer, an option is to discontinue the operation and treat the patient with radiation therapy (71). If the lymph nodes are negative for disease, the left side of the aorta is palpated through the peritoneal incision with a finger passed under the inferior mesenteric artery. The lymph nodes on this side of the aorta are more lateral and nearly behind the aorta and the common iliac artery. If the left para-aortic lymph nodes appear healthy and the cervical tumor is small with no suspicious pelvic lymph nodes, these additional lymph nodes are not submitted for frozen-section analysis. If they are removed, they may be dissected through the incision made for the right para-aortic nodes, or they may be dissected after reflection of the sigmoid colon medially.
Development of Pelvic Spaces
The pelvic spaces are developed by sharp and blunt dissection (Fig. 36.8).
The paravesical space is bordered by the following structures:
The attachments of the vagina to the tendinous arch form the floor of the paravesical space.
The pararectal space is bordered by the following structures:
The coccygeus (levator ani) muscle forms the floor of the pararectal space.
The development of these spaces before pelvic lymphadenectomy will aid in identification and dissection of the pelvic lymph nodes and dissection of the ureter as it passes into the vesicouterine ligament tunnel.
Dissection of the Bladder
The dissection of the bladder from the anterior part of the cervix and vagina is a critical step. Occasionally, tumor extension into the base of the bladder (which cannot be detected with cystoscopy) precludes adequate mobilization of the bladder flap, leading to the abandonment of the operation. Therefore, this portion of the operation should be undertaken early in the procedure. The bladder should be mobilized off of the upper third of the vagina to remove the tumor safely and with adequate margins.
Dissection of the Uterine Artery
The superior vesicle artery is dissected away from the cardinal ligament at a point near the uterine artery. The uterine artery, which usually arises from the superior vesicle artery, is thus isolated and divided, preserving the superior vesicle arteries. The uterine vessels are brought over the ureter by application of gentle traction. Occasionally, the uterine vein passes under the ureter.
Dissection of the Ureter
The ureter is dissected free from the medial peritoneal flap at the level of the uterosacral ligament. As the ureter passes near the uterine artery, there is a consistent arterial branch from the uterine artery to the ureter. This branch is sacrificed in the standard radical (type III) hysterectomy but preserved in the modified radical (type II) hysterectomy. Dissection of the ureter from the vesicouterine ligament (ureteral tunnel) may now be accomplished. If the patient has a deep pelvis, ligation of the uterosacral and cardinal ligaments may be undertaken first to bring the ureteral tunnel dissection closer to the operator. The roof of the ureteral tunnel is the anterior vesicouterine ligament. It should be ligated and divided to expose the posterior ligament. The posterior ligament is divided in the radical (type III) hysterectomy but conserved in the modified radical (type II) hysterectomy.
Posterior Dissection
The peritoneum across the cul-de-sac is incised, exposing the uterosacral ligaments. The rectum is rolled free from the uterosacral ligaments, which are divided midway to the sacrum in a radical (type III) hysterectomy and near the rectum in the modified radical (type II) operation. This allows the operator to isolate and separate the cardinal ligament from the rectum. A surgical clamp is placed on the cardinal ligament at the lateral pelvic sidewall in a radical hysterectomy and at the level of the ureteral bed in the modified radical procedure. A clamp is placed on the specimen side to maintain traction and to ensure that the full cardinal ligament is excised with the specimen. A right-angled clamp is placed caudad to this clamp across the paravaginal tissues. A second paravaginal clamp is usually needed to reach the vagina.
The vagina is entered anteriorly, and a suitable margin of proximal vagina is removed with the specimen. More vaginal epithelium can be excised if necessary, depending on the previous colposcopic findings. The vaginal edge may be sutured in a hemostatic fashion and left open with a drain from the pelvic space or closed with a suction drain placed percutaneously. The ureteral fistula and pelvic lymphocyst rates from these two techniques are similar.
Complications of Radical Hysterectomy
Acute Complications
The acute complications of radical hysterectomy include (74):
Febrile morbidity is most often caused by pulmonary infection (10%) and is seen frequently with pelvic cellulitis (7%) and urinary tract infection (6%). Wound infection, pelvic abscess, and phlebitis all occur in fewer than 5% of patients (75).
Subacute Complications
The subacute effects of radical hysterectomy are postoperative bladder dysfunction and lymphocyst formation. For the first few days after radical hysterectomy, bladder volume is decreased, and filling pressure is increased. The sensitivity to filling is diminished, and the patient is unable to initiate voiding. The cause of this dysfunction is unclear. It is important to maintain adequate bladder drainage during this time to prevent overdistention. Bladder drainage is usually accomplished with a suprapubic catheter. It is more comfortable for the patient and allows the physician to perform cystometrography and determine residual urine volume without the need for frequent catheterization. In addition, the patient is able to accomplish voiding trials at home by clamping the catheter, voiding, and releasing to check the residual urine level. Cystometrography may be performed 3 to 4 weeks after surgery. For the catheter to be discontinued, the patient must be able to sense the fullness of the bladder, initiate voiding, and void with a residual urine level of less than 75 to 100 mL. Otherwise, voiding trials should continue at home until these criteria can be fulfilled.
Lymphocyst formation occurs in fewer than 5% of patients, and the cause is uncertain (75). Adequate drainage of the pelvis after radical hysterectomy may be an important step in prevention. However, routine placement of retroperitoneal drains did not reduce this morbidity (73). Ureteral obstruction, partial venous obstruction, and thrombosis may occur from lymphocyst formation. Simple aspiration of the lymphocyst is generally not curative, but percutaneous catheters with chronic drainage may allow healing. If this treatment is unsuccessful, operative intervention with excision of a portion of the lymphocyst wall and placement of either large bowel or omentum into the lymphocyst should be performed.
Chronic Complications
The most common chronic effect of radical hysterectomy is bladder hypotonia or, in extreme instances, atony. This condition occurs in about 3% of patients, regardless of the method of bladder drainage used (76,77). It may be a result of bladder denervation and not simply a problem associated with bladder overdistention (78). Voiding every 4 to 6 hours, increasing intra-abdominal pressure with Credé’s maneuver, and intermittent self-catheterization may be used to manage bladder hypotonia.
Ureteral strictures are uncommon in the absence of postoperative radiation therapy, recurrent cancer, or lymphocyst formation (78). If the stricture is associated with lymphocyst formation, treatment of the lymphocyst usually alleviates the problem. Strictures that occur after radiation therapy should be managed with ureteral stenting. If a ureteral stricture is noted in the absence of radiotherapy or lymphocyst formation, recurrent carcinoma is the most common cause. A CT scan of the area of obstruction should be obtained and cytologic assessment by FNA should be performed if there is a target lesion to exclude carcinoma. If the results of these tests are negative, a ureteral stent may be placed to relieve the stricture. Close observation for recurrent carcinoma is necessary, and the diagnosis of recurrence may ultimately require laparotomy.
Nerve-Sparing Radical Hysterectomy
Nerve-sparing radical hysterectomies were described in recent years in an attempt to diminish the bladder dysfunction, sexual dysfunction, and colorectal motility disorders commonly encountered after traditional radical hysterectomy. Multiple techniques were described involving the identification of the pelvic autonomic nerves at the sacral promontory followed by various surgical methods of nerve preservation as the nerves transit the cardinal ligaments. These techniques are promising and in small series did reduce postoperative bladder dysfunction (79,80).
Laparoscopic Radical Hysterectomy
Laparoscopic-assisted radical vaginal hysterectomy is being performed with increasing frequency in highly selected patients. In one large series of 200 women with stages IA1 to IIB cervical cancer treated with laparoscopic lymphadenectomy followed by radical vaginal hysterectomy, the authors found a 5-year survival rate comparable to patients treated with a similar abdominal approach and a comparable rate of intraoperative complications (81).
The use of laparoscopy in cervical cancer patients is appealing because it may lead to less blood loss, improved cosmetic results, shorter duration of hospitalization, and faster recovery.
Robotic Laparoscopic Radical Hysterectomy
Robotic laparoscopic radial hysterectomy is a relatively new technique. Proponents argue that in highly selected patients it can decrease hospital admission time and decrease the surgical morbidity in obese patients. One study reports comparable body mass index, operative times, parametrial margin, and number of lymph nodes collected when compared to open cases. Robotic cases had significantly shorter hospital stays and blood loss, while having significantly larger incidence of postoperative bladder dysfunction. The technique is still too new to tabulate cancer outcome data (82,83).
Sentinel Lymph Node Evaluation
Sentinel lymph node detection has become an integral part of the management strategy for breast cancer and melanoma and is being investigated as a diagnostic tool in multiple human malignancies, including carcinoma of the cervix. The sentinel node is a specific lymph node (or nodes) that is the first to receive drainage from a malignancy and is a primary site of nodal metastasis. In theory, the presence or absence of metastatic disease in the sentinel node should reflect the status of the nodal basin as a whole. Thus, a negative sentinel lymph node would allow omission of lymphadenectomy of the involved nodal basin. Sentinel lymph nodes are detected through perilesional injection of radiolabeled technicium-99 or blue dye followed by intraoperative identification of the sentinel lymph nodes utilizing handheld gamma probes or visual identification of blue-stained nodes. These techniques are primarily applicable in patients with early-stage disease and clinically negative lymph nodes, in whom lymph node status may influence the extent of the procedure or the use of adjuvant treatment.
Although data utilizing sentinel lymph node detection techniques in cervical cancer are limited, several interesting conclusions can be drawn from completed studies. Sentinel nodes can be detected in 80% to 100% of cervical cancer patients, and these rates were confirmed by both laparotomy and laparoscopy. A combination of dye and radiolabeled techniques appears to be superior for the detection of sentinel lymph nodes over either technique used alone. Test sensitivity of 65% to 87% can be expected with a 90% to 97% negative predictive value. The likelihood of detecting sentinel nodes may depend on the tumor volume, the time from injection to retrieval of the sentinel nodes, and the volume of dye or radiolabeled tracer injected. Sentinel node detection rates do not appear to be influenced by prior cold knife cone biopsy. False-negative results were reported. The role of sentinel node detection in cervix cancer is investigational; although the technique is promising, complete lymphadenectomy, when indicated, remains the standard of care (84).
Postoperative Management
Prognostic Variables for Early-Stage Cervical Cancer (Ia2–IIa)
The survival of patients with early-stage cervical cancer after radical hysterectomy and pelvic lymphadenectomy depends on the presence or absence of several intermediate and high-risk pathologic factors (76,85–99).
Intermediate risk factors for recurrent disease are:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


