FIGURE 11.1 Sagittal (left panel) and coronal (right panel) magnetic resonance venograms demonstrating normal venous sinuses. (a) Superior sagittal sinus. (b) Internal cerebral veins. (c) Great vein of Galen. (d) Straight sinus. (e) Confluence of sinuses (torcula). (f) Left transverse sinus. (g) Left sigmoid sinus. (h) Left internal jugular vein. Note that the Inferior sagittal sinus is not visible in this figure.
Superficial system: The superior sagittal sinus (SSS) is confined by the attached borders of the falx cerebri. Blood from the medial and lateral surfaces of the frontal, parietal, and occipital lobes as well as from the scalp drains into the SSS. Cutaneous infections or contusions may cause SSS thrombosis. An anatomical variant exists in which the anterior portion of the SSS may be replaced by two superior cerebral veins that join behind the coronal suture. The SSS terminates at the junction of the straight and transverse sinus which is referred to as the confluence of the sinuses or torcula herophili. One transverse sinus, more often the right, may be dominant with mild or greatly diminished flow in the contralateral sinus (12–14). This is thought to be related to the more linear course from the right internal jugular vein to the superior vena cava and right atrium. Isolated lack or diminution of flow-related signal in one transverse sinus on time-of-flight magnetic resonance venogram (MRV) is typical in individuals with unilateral hypoplasia of the transverse sinus and should not be mistaken for a diagnosis of CVST. The inferior sagittal sinus lies in the falx cerebri free edge and drains into the straight sinus.
Deep system: The straight sinus is formed by the tentorium cerebelli and drains the vein of Galen and inferior sagittal sinus. It joins the torcula at the occipital protuberance. The transverse sinuses are formed from the attached borders of the tentorium cerebelli and drain blood from the posterior cerebral hemispheres, cerebellum, and brainstem into the sigmoid sinuses. The sigmoid sinuses drain the transverse sinuses into the internal jugular veins. The position of the sigmoid sinus over the inner aspect of the mastoid bone predisposes it to thrombosis with inner ear or mastoid infection (15).
Other: The cavernous sinuses are two large channels on either side of the sella turcica. The abducens nerve and internal carotid artery (carotid siphon) are located within the center of each cavernous sinus, and the oculomotor nerve, trochlear nerve, and ophthalmic and maxillary portions of the trigeminal nerve run along the lateral wall of each cavernous sinus. The cavernous sinuses drain the ophthalmic veins and the anterior base of the brain via the middle cerebral vein. Infection of the face or sphenoid sinus predisposes to thrombosis of the cavernous sinuses. The superior and inferior petrosal sinuses drain the cavernous sinuses into the transverse and sigmoid sinuses, respectively. The thalamostriate veins drain the posterior frontal and anterior parietal lobes, the caudate nucleus, and the internal capsule. Deep structures of the frontal lobe drain into the basal vein of Rosenthal. The internal cerebral veins and basal vein of Rosenthal join to form the vein of Galen which drains into the straight sinus. Malformation of the vein of Galen results in arteriovenous shunting of blood and aneurysmal dilation of the vein and can be associated with thrombosis.
Anastomoses: The vein of Trolard connects the middle cerebral vein to the SSS. The vein of Labbé connects the middle cerebral veins to the transverse sinus. Anastomoses explain the absence of well-defined venous territories, and hence, well-defined clinical syndromes associated with thrombosis of a particular sinus or vein.
Sinuses most commonly involved: The most commonly involved sinuses with CVST are the SSS in 23% to 72% of cases (4,9,10,16–22), the transverse sinus in 6% to 74% of cases (4,9,10,16–22), the straight sinus in 13% to 33% of cases (9,17,18,20,21), and the internal veins in 1% to 8% of cases (4,9,17,20,21). Head computed tomography (HCT) is less sensitive for deep vein thrombosis, and thrombosis of deep veins may therefore be underrecognized (18,21). Involvement of multiple sinuses is common and is seen in 14% to 70% of cases (1,4,9–11,18,20–23).
■ PATHOPHYSIOLOGY
Many acute and chronic conditions are risk factors for CVST (Table 11.1). The cerebral veins and dural sinuses are common sites for thrombosis when one or multiple risk factors are present since blood flow is slow and follows a tortuous route. The presence of thrombosis causes venous outflow obstruction and creates a high pressure venous system that rivals the pressure in the arterial system. Capillary hydrostatic pressure increases as a result. Arterial perfusion may be inadequate when venous pressure exceeds arterial pressure, and ischemia may result. A high capillary hydrostatic pressure drives plasma filtrate into the interstitium leading to parenchymal edema. Thrombus in the dural sinuses, especially the SSS, may impair cerebrospinal fluid absorption through arachnoid villi and granulations (pacchionian bodies) that protrude into these sinuses, resulting in communicating hydrocephalus. Parenchymal findings reflect the pathophysiology and include edema, hemorrhage, and ischemia.

■ CLINICAL MANIFESTATIONS
The clinical manifestations of CVST are nonspecific and variable. The classical presentation of CVST is a triad of headache, vomiting, and depressed level of consciousness. CVST can present suddenly with acute severe headache and rapid deterioration of consciousness or may develop insidiously over days to weeks.
In the largest multinational prospective observational study of CVST in adults, the evolution of clinical symptoms was acute in 37%, subacute in 56%, and chronic in 7% (24). Reflecting this variable course of symptom evolution, the time of symptom onset to hospital presentation in children ranges from 0 to 120 days. The median time to hospital presentation in children is 4 to 5 days (11,25), and the majority of children present within the first week (11,28). Delay in diagnosis often occurs, perhaps due to symptoms that are both nonspecific and often subtle.
Symptoms and signs associated with CVST are also associated with many other diagnoses that include arterial ischemic stroke, intracerebral hemorrhage, mass lesions such as brain tumor or abscess, meningitis, hydrocephalus, and idiopathic intracranial hypertension. The range of symptoms reported in children is broad (1–4,7,10,11,16,17,21,25–27). They include headache (21%–95%), nausea or emesis (21%–67%), visual symptoms (11%–38%), papilledema (22%–76%), cranial nerve abnormalities (10%–35%) often involving the sixth nerve (abducens nerve), hemiparesis (3%–33%), ataxia (6%–7%), aphasia (13%), and hemisensory loss. Fever is a common finding, seen in 29%–58% and may reflect the high association of CVST with infection. Seizures are reported in 20% to 48% of children, depressed consciousness or lethargy in 11% to 55%, and coma in 4% to 28% (1–4,7,10,11,16,17,21,25–27). Finally, CVST can be completely asymptomatic, found only when imaging is performed for another reason or because the patient has a known risk factor for CVST such as mastoiditis.
The involved sinuses cannot usually be distinguished by clinical features alone, but certain observations have been made. A review of 52 children with CVST found that depressed consciousness was common when the straight sinus was involved. Involvement of the SSS was observed with depressed consciousness, seizures, and focal signs. Papilledema with sixth nerve palsy occurred with thrombosis associated with mastoiditis (21).
■ RISK FACTORS
A risk factor for CVST is identified in 74% to 95% of children, and at least half of children affected by CVST have multiple risk factors (10,11,17,21,25). Table 11.1 lists risk factors for CVST.
Infections, particularly of the head and neck, are among the most common predisposing conditions, reported in 50% to 57% of children (2,3,7,10,11,21,22,23,25,26). These include otitis media or mastoiditis in 9% to 62% and sinusitis in 2% to 36% (Figure 11.2). An intracranial infection, such as meningitis or intracranial abscess, is reported in 11% to 44%, and an extracranial infection in 24% to 33% of children. Sepsis is found more frequently in association with neonatal thrombosis but can also be a risk factor in childhood.
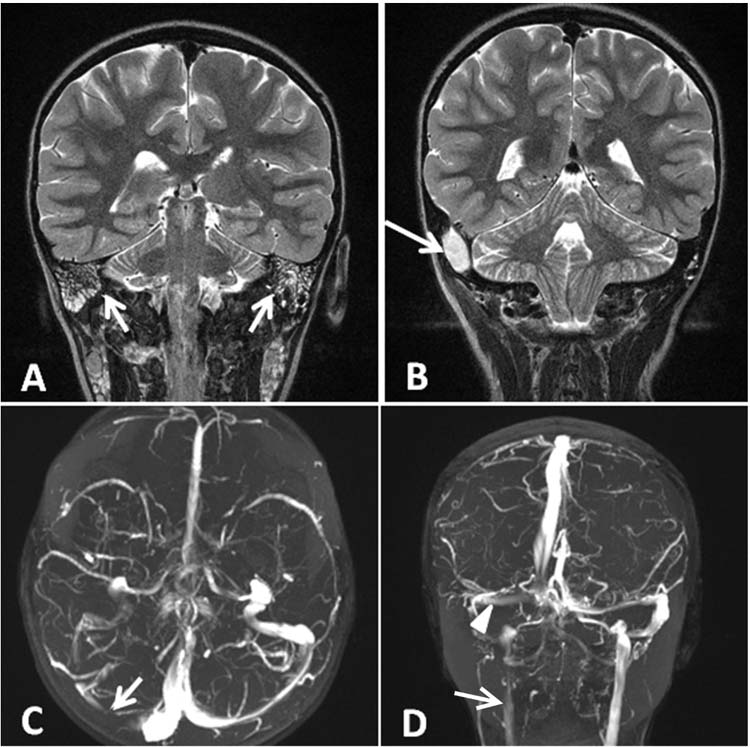
FIGURE 11.2 (A) Coronal T2-weighted magnetic resonance imaging (MRI) with bilateral mastoiditis (arrows). (B) Coronal T2-weighted MRI also demonstrates a right sided epidural abscess as a complication (arrow). (C) Axial time-of-flight MRV with decreased flow in the right transverse sinus (arrow) due to thrombus. (D) Coronal MRV again demonstrating decreased flow in the right transverse sinus (arrowhead) and merely a trickle of flow in the right internal jugular vein (arrow).
Dehydration and anemia are consistently described in children with CVST (11,17,23–25). Dehydration increases blood viscosity and is reported in 5% to 28%. The pathophysiology of the association between anemia and cerebral venous sinus thrombosis is not well understood and yet is reported in 10% to 19% of children. In one series, thrombocytosis was reported as a risk factor (25); however, thrombocytosis is a well-established physiologic response to anemia. It is not clear whether thrombocytosis is an independent risk factor for CVST in the absence of anemia.
The postsurgical state, in particular recent cranial surgery, is reported in 6% to 9% of children in two series (2,11). Recent head trauma is described in 4% to 6% (2,11,17). Closed head injury without skull fracture has also been reported as a risk factor (29).
Pregnancy and the puerperium are times of increased risk for CVST, seen in 14% and 6% of adults with CVST (24). Several medications have been associated with CVST. Oral contraceptive use is a well-established risk factor for thrombosis in adults and is reported as a risk factor in children (1,25). Corticosteroids have also been associated with CVST (30).
Certain chronic diseases have been implicated as risk factors for CVST and are present in 34% to 60% of affected children (1,3,7,11,17,21). Reported underlying conditions include hematologic malignancy, nephrotic syndrome, cardiac disease, and inflammatory conditions such as inflammatory bowel disease, celiac disease, systemic lupus erythematosus, and Behçet’s disease.
Leukemia or lymphoma is reported in 5% to 18% of childhood CVST (3,7,17). The high blood viscosity conferred by hematologic malignancy may be important, and treatment with L-asparaginase increases risk (Figure 11.3). L-asparaginase, a chemotherapeutic often used to treat acute lymphoblastic leukemia (31), has been associated with CVST (31,32), possibly related to its effect on the synthesis of coagulation factors and inhibitors of coagulation such as proteins C and S. Nephrotic syndrome is rare in children but has been reported in 4% of children with CVST (11,21,23,25). Children with nephrotic syndrome have been found to have lower antithrombin III levels than healthy controls (33). Other potential contributors to a prothrombotic state in children with nephrotic syndrome are abnormal fibrinogen levels, abnormal lipid profiles, and reduced intravascular volume. A review of children with nephrotic syndrome found that when CVST was a complication, it was present with the first flare or within the first 6 months of diagnosis (34). Inflammatory conditions like systemic lupus erythematosus are seen in 4% to 7% of children with CVST (11,23). In systemic lupus erythematosus, secondary antiphospholipid syndrome characterized by anticardiolipin antibodies and/or lupus anticoagulant predisposes the child to venous and arterial thrombosis. A thrombotic event including CVST can herald the presentation of systemic lupus erythematosus (35). Behçet’s disease is a vascular inflammatory disease with prominent venous involvement. The frequency of nervous system involvement was 13% among males and 6% among females in a 2-decade prospective cohort (36). In a series of children with Behçet’s, 52% of thromboses were intracranial (37). In one series of children with CVST, 7% had Behçet’s (3).
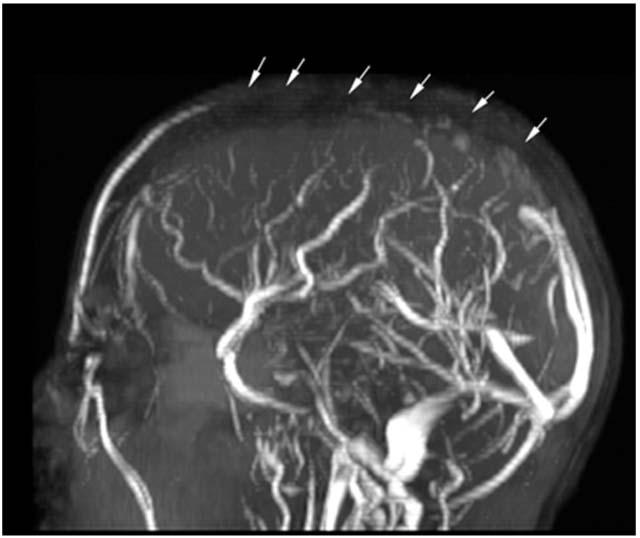
FIGURE 11.3 Sagittal MRV with decreased flow in the superior sagittal sinus (arrows) representing thrombus in a 17-year-old with acute lymphoblastic leukemia 10 days after receiving L-asparaginase.
A prothrombotic risk factor has been reported in 40% to 76% of children with CVST (4,11,13,17,19,21,23,38,39,40). In series that differentiate between children and neonates, a prothrombotic abnormality was found in 34% to 62% of children. The absence of standardized prothrombotic testing across institutions may account for these widely variable estimates of prevalence. In a large series of 149 children and neonates with CVST, univariate analysis showed a significantly higher prevalence of factor V G1691A mutation, elevated lipoprotein (a), protein C deficiency, and protein S deficiency in children with CVST compared to controls. However in multivariate analysis, only elevated lipoprotein (a), protein C deficiency, and a combination of a prothrombotic factor and another risk factor were independently associated with CVST (4). A recent large meta-analysis demonstrated significant associations between first CVST and prothrombotic risk factors. Prothrombotic factors with the highest association with CVST were antithrombin III deficiency, protein C deficiency, protein S deficiency, or the presence of > 2 prothrombotic traits. A significant though weaker association was present for factor V G1691A and factor II G20210A (41). In recent years, elevated factor VIII level has emerged as a possible risk factor for CVST (11), and both elevated factor VIII level and D-dimer levels have been reported in association with systemic thromboembolism (42,43).
■ DIAGNOSTIC STUDIES
Unenhanced HCT is routinely the first diagnostic test employed to screen for an acute intracranial process in children presenting with symptoms of CVST. In pediatric series, the sensitivity of unenhanced HCT for findings suggestive of CVST is 69% to 88% (2,22,25). Primary signs of CVST are the cord sign, the dense triangle sign, and the empty delta or empty triangle sign (Figure 11.4). The cord sign is visualized dense cortical veins or thrombosed sinuses. The dense triangle sign is a very early and transient sign. It is visualization of acutely congealed blood in the SSS. The delta or empty triangle sign is a late finding, requires contrast, and is the visualization of normally opacified collateral veins in the walls of the sinus, usually of the SSS, juxtaposed to nonopacified thrombus. In one series of 19 children, an empty delta sign was seen in 100%, a dense triangle sign in 42%, and a cord sign in only one subject (27).
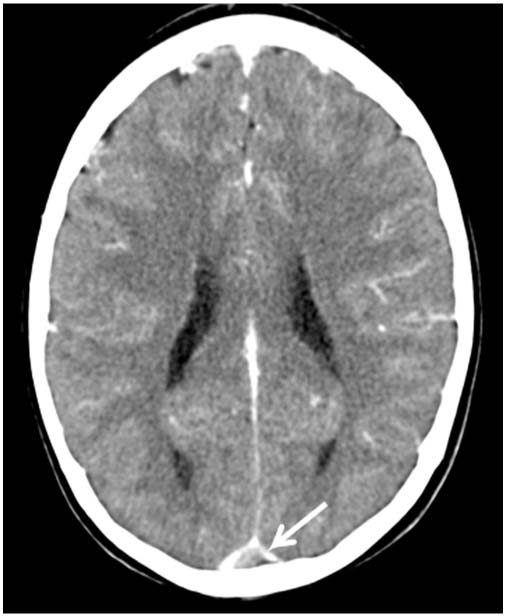
FIGURE 11.4 Contrast enhanced head computed tomography (HCT) demonstrating the empty delta or triangle sign (arrow) in a child with nephrotic syndrome and superior sagittal sinus thrombosis. There is low-attenuating thrombus within the superior sagittal sinus surrounded by a triangular area of enhancement.
On acute HCT, parenchymal effects of a CVST can sometimes be visualized. Early imaging findings may be restricted to vasogenic and cytotoxic edema. Isolated ischemic infarction was seen in 17% of children in one series (Figure 11.5) (16). Hemorrhage may be more common than isolated ischemia and has been reported in 16% to 23% of children (1,3,16,27). Hemorrhage can be parenchymal, intraventricular, or subarachnoid (16). Intraventricular hemorrhage is rare in children with CVST, reported in only 2% in two series (3,16). Hemorrhage on HCT along the transverse sinuses, in the biparietal region, or in the bithalamic region should prompt further evaluation for CVST, even if no clot is apparent because hemorrhage can obscure findings of the CVST itself (Figure 11.6). An absence of parenchymal findings has been reported in 14% to 26% of children (2,16). Children with polycythemia can have HCT findings that are difficult to differentiate from those present in CVST, so confirmatory vascular imaging should be performed quickly in most cases of suspected CVST so that appropriate treatment decisions can be made.
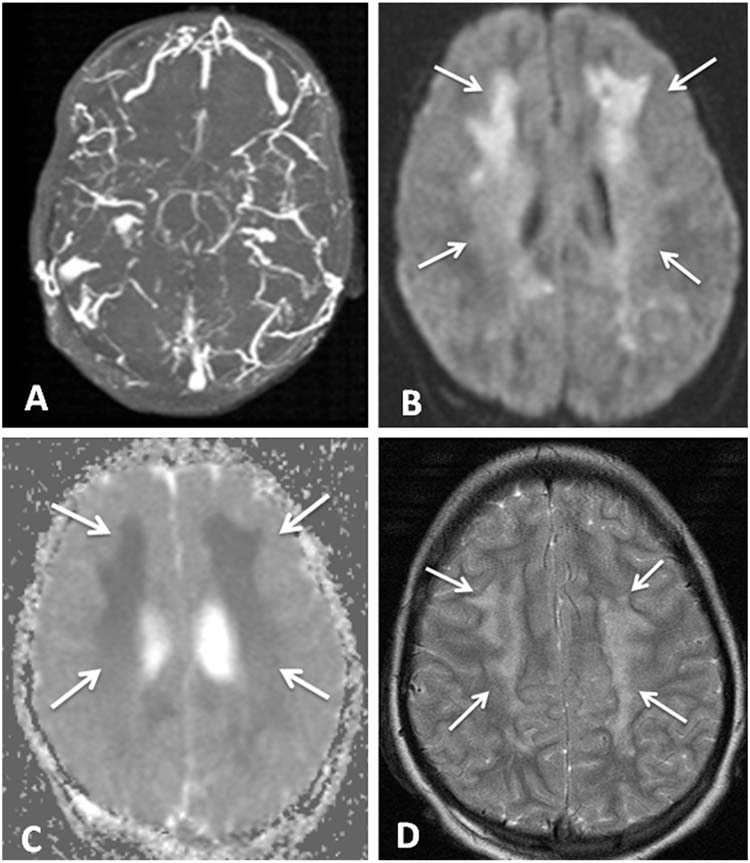
FIGURE 11.5 (A) Axial MRV with diffuse cerebral venous sinus thrombosis. (B) Axial diffusion weighted image with restricted diffusion representing ischemia in the white matter bilaterally (arrows). (C) Dark areas (arrows) on axial apparent diffusion coefficient map corresponding to areas of restriction on DWI confirm ischemia. (D) Axial T2-weighted image with hyperintensities (arrows) corresponding to the areas of abnormality on DWI and ADC.
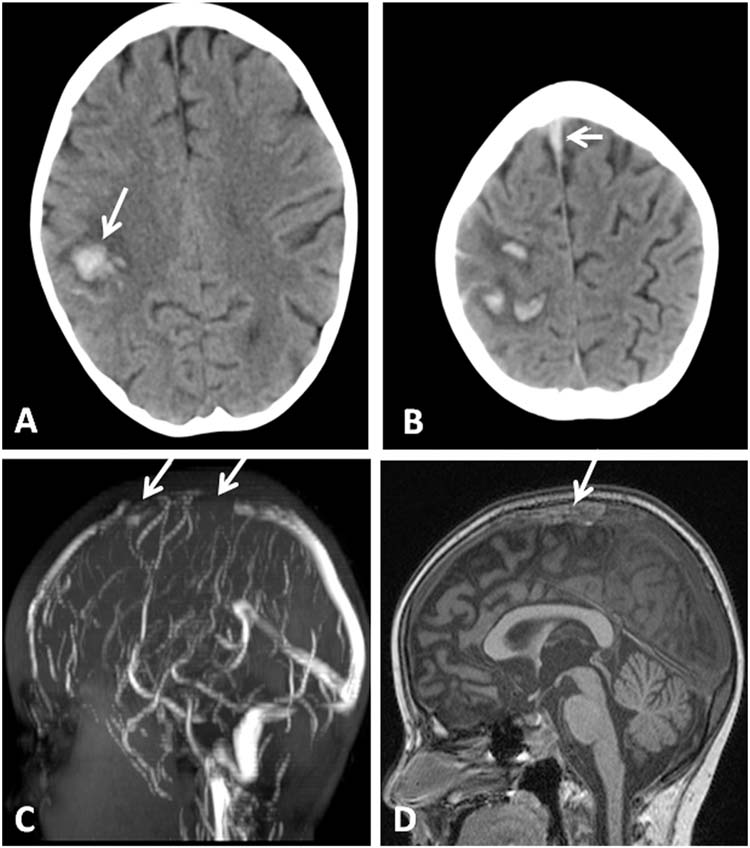
FIGURE 11.6 (A) HCT with hemorrhage in right frontal lobe (arrow) in a 6-year-old girl with acute lymphoblastic leukemia recently treated with L-asparaginase. (B) Higher on the same HCT, hyperdensity representing clot in the superior sagittal sinus is noted (arrow). (C) Sagittal MRV with flow void in anterior superior sagittal sinus representing thrombus (arrows). (D) Sagittal T1-weighted MRI with hyperintensity in area of anterior superior sagittal sinus representing thrombus (arrow).
Confirmatory tests include magnetic resonance imaging (MRI) performed concomitantly with MRV, computed tomography venogram (CTV), and four-vessel conventional angiography with venous phase. An advantage of the combination of MRI and MRV is the absence of radiation and contrast exposure, a high sensitivity for venous thrombosis, and the visualization of secondary parenchymal changes. Intraluminal thrombus is visualized on T2-weighted MRI as absence of a flow void or on T1-weighted MRI as hyperdense signal. The limitations of MRI are related to properties of blood flow and to the evolution of signal for thrombus over time. When blood flow is slow or the direction of blood flow is in the imaging plane, signal intensity is diminished and can be mistaken for thrombus. The appearance of thrombus changes with maturation of the clot. Within the first 5 days, deoxyhemoglobin is isointense on T1-weighted images and is hypointense on T2-weighted images. Deoxyhemoglobin can be mistaken for flowing blood. In the subacute phase, at 5 to 15 days, methemoglobin, which is easier to visualize, is hyperintense on both T1- and T2-weighted images (44). Therefore discussion with the neuroradiologist about the timing of the patient’s presentation is helpful. Despite limitations, the combination of MRI and MRV has high sensitivity for CVST. In one study, 100% of children with CVST had a diagnostic MRI/MRV (25).
CTV has the advantage of rapid acquisition time, typically less than 1 minute, and can be used in children with medical devices that are not MRI compatible. However, drawbacks include radiation exposure and the risk of contrast nephropathy. Additionally, the quality of the study is related to appropriate timing between contrast administration and the image acquisition. At many centers the radiation dose for CTV (1–2.22 mSv) can be equivalent to or even lower than the radiation dose of unenhanced HCT (2.48 mSv) (45). On CTV, thrombosis is visualized as a filling defect, similar to the findings on conventional angiography with venous phase. When CVST is present, the time from administration of contrast to visualization of contrast in the cerebral veins and sinuses is delayed on the order of seconds (46). Many centers adjust their delay time for suspected CVST, but optimal delay time has not been established. In one study, the sensitivity and specificity of high resolution helical multidetector-row CT was found to be 100% (47).
When comparing MRI/MRV to CTV for the visualization of thrombosis, more than one study has demonstrated that both tests can establish the diagnosis well. CTV is superior to MRV for the visualization of smaller venous structures with low flow (48,49). MRI is superior to HCT for visualization of parenchymal changes secondary to cerebral venous thrombosis.
Four-vessel conventional angiography with venous phase is considered the gold standard test for CVST, but with updated CT and MR techniques, conventional angiography is rarely needed to make the diagnosis.
■ LABORATORY EVALUATION
In the acute setting, a complete blood count (CBC) with differential and platelets, activated partial thromboplastin time (aPTT), prothrombin time (PT)/international normalized ratio, and basic metabolic panel (BMP) should be sent. The CBC can help screen for infection, anemia, and thrombocytosis. Fibrinogen levels may be useful if the PTT or PT is abnormal. D-dimer is often but not always elevated in the setting of acute thrombus. The BMP can help assess for dehydration and electrolyte abnormalities such as hypernatremia.
A complete evaluation for thrombophilia is recommended in the American Heart Association (AHA) guidelines for the management of stroke in infants and children (50), but this recommendation has Class IIb/Level B evidence. The combination of 2 prothrombotic factors or a prothrombotic factor plus another CVST risk factor is common (11), so evaluation for thrombophilia may be warranted even when another CVST risk factor is present. Thrombophilia evaluation typically includes protein C and protein S levels, antithrombin III level, homocysteine level, lipoprotein (a), antiphospholipid antibodies (immunoglobulin G and immunoglobulin M), dilute Russell venom viper time, and genetic testing for factor V G1691A mutation and factor II G20210A variant. Some institutions also measure factor VIII levels. Prior to the initiation of anticoagulation, antithrombin III level must be sent since this level is altered once heparin is started. In the case of a prothrombotic abnormality, the laboratory test is often repeated weeks to months after the acute thrombus since the presence of thrombus itself can lower levels of certain factors such as proteins C and S. Consultation with a hematologist is strongly recommended to assist with interpretation of thrombophilia studies and with treatment. In children with anemia, a comprehensive diagnostic evaluation and treatment of the anemia are important.
If infection is suspected, a source should be investigated. Examination of the ears is part of the routine evaluation for all children. Sinus radiographs and blood cultures should be considered when there is suspicion for sinusitis and sepsis, respectively. Lumbar puncture with measurement of opening pressure and cerebrospinal fluid analysis should be considered in children who present with clinical signs of an intracranial infectious process. However, this may not be possible if there is evidence of mass effect or signs of greatly increased intracranial pressure. Even in CVST that is not associated with intracranial infection, cerebrospinal fluid analysis may be abnormal. In a series of 25 neonates and children with CVST, 19 underwent lumbar puncture. Elevated protein was present in 37% and subarachnoid hemorrhage was present in 79%. Opening pressure was measured in five and was elevated in four (26).
■ MANAGEMENT
Management of CVST focuses on providing supportive care, preventing and assessing for neurologic deterioration, and preventing propagation of thrombus.
Supportive Measures
It is crucial that patients with CVST be hydrated with isotonic fluids, and fluids are often administered at 1.5 times maintenance for at least 24 to 48 hours unless there is a contraindication. Very young infants may require hypotonic fluids, but this is usually not necessary after the neonatal period. Normoglycemia and normothermia are also goals. The head should be positioned at 30º or higher to promote venous outflow which may help to decrease intracranial pressure (ICP). If a bacterial etiology is suspected, broad spectrum intravenous antibiotics, with anaerobic coverage for possible sinus disease, should be initiated. For severe otitis media or mastoiditis, procedures including mastoidectomy, myringotomy, and/or tympanostomy may be indicated in addition to antibiotics. If an epidural abscess is present, neurosurgical drainage is often required.
Monitoring for and Treatment of Elevated ICP
In the acute phase, frequent neurologic examination is imperative. The clinician should pay particular attention to signs of elevated ICP including headache, emesis, hypertension, bradycardia, and deteriorating mental status including agitation and combativeness. Papilledema and changes in visual acuity, visual fields deficits, cranial nerve deficits (particularly nerves III and VI), and new motor deficits may also indicate elevated ICP or new parenchymal brain injury. In any child with an acute neurologic change, a noncontrast HCT should be obtained immediately.
Management of intracranial hypertension in children with CVST and cerebral edema or mass effect from hemorrhage is highly complex. The usual treatment strategies, including invasive ICP monitoring, hyperosmolar therapy, ventricular drainage, or decompressive surgery, must be used judiciously, balancing the risk of hemorrhagic complications and the need for suspending anticoagulation compared to the anticipated benefit.
Acetazolamide may be helpful in decreasing ICP since the medication is a carbonic anhydrase inhibitor and can decrease the production of cerebrospinal fluid; however, the medication will not acutely lower ICP in an emergency. There is limited literature on decompressive craniectomy for impending herniation in the setting of CVST. Case reports describe adult patients with large hemorrhagic infarcts accompanying CVST with signs of herniation that underwent successful decompressive craniectomy (51). A pediatric series also describes children who underwent decompression (22).
Monitoring for and Treatment of Seizures
There is concern that the metabolic demand of a seizure might worsen the extent of brain injury. If the child presents with a seizure, an antiepileptic medication should be considered. However, prophylactic anticonvulsants have not been studied in children with CVST. In patients who are comatose, sedated or paralyzed, or intubated, continuous electroencephalographic (EEG) monitoring should be strongly considered to screen for subclinical seizures (50). Other children may require continuous EEG monitoring including those with fluctuating mental status or who presented with seizures. In most cases even electrographic seizures without clinical manifestations are treated. In one series in which 8 of 15 children were monitored with EEG, it was abnormal in 75% due to diffuse slowing (26).
Anticoagulation
For many years the use of anticoagulation was considered counterintuitive in CVST because of the risk of concomitant hemorrhagic infarction. While there continues to be concern about the use of anticoagulation, especially in patients with large hemorrhages, treatment with anticoagulation is recommended in most cases of pediatric CVST (50,52). While there are no randomized controlled trials for anticoagulation in the pediatric population, recommendations supporting the use of anticoagulation in children are based on two randomized controlled trials in adult CVST (53,54) and experience from pediatric cohorts (9,16,23). The first adult trial comparing acute unfractionated heparin versus placebo was stopped early because complete clinical recovery at 3 months was seen in 80% of the heparin group compared with 10% of the placebo group (53). A larger blinded randomized controlled trial was conducted comparing acute treatment with low molecular weight heparin (LMWH) to placebo. A poor outcome (death or dependence) was seen in 13% of the anticoagulation group compared with 21% of the placebo group. These findings were not statistically significant despite a larger cohort (54). Both adult trials included patients with hemorrhage accompanying the CVST. In a Cochrane systematic review that pooled the results of the two adult randomized controlled trials, the relative risk of death in those treated with anticoagulation compared to placebo patients was 0.33 (95% confidence interval [CI] 0.08–1.21), and the relative risk of dependency was 0.46 (95% CI 0.16–1.31) (55). The pooled estimates did not reach statistical significance; however, no new symptomatic intracranial hemorrhages occurred in the patients treated with anticoagulation in either trial. The authors of the systemic review recognized that additional clinical trials would be difficult and concluded that anticoagulation in the setting of CVST seemed to be safe and had potential for reducing mortality and dependence (55).
Pediatric cohort studies also suggest that anticoagulation in the setting of CVST is safe. In one study of 30 children with CVST, 22 children without concomitant hemorrhage were treated with anticoagulation. Only one of these children had an intracranial hemorrhage after anticoagulation was initiated, and that hemorrhage was asymptomatic (23). In that same cohort, three deaths occurred among the 8 children who did not receive anticoagulation. In a larger cohort of children from the Canadian Stroke Registry, 66% of the children received antithrombotic therapy, most of which was anticoagulation. None of the children on anticoagulation died or experienced neurologic worsening due to hemorrhagic complications (1). In a more recent cohort study, 3 of 61 children treated with anticoagulation had major hemorrhages, but none with anticoagulation-related hemorrhage died. Two of these major hemorrhages occurred in children with intracranial hemorrhage prior to anticoagulation, and one occurred in a child without intracranial hemorrhage prior to anticoagulation. This study also demonstrated a statistically significant decrease in propagation of thrombus in children treated with anticoagulation compared to those who were not treated with anticoagulation. While not statistically significant in multivariate analysis, in univariate analysis anticoagulation was associated with favorable outcome compared to children who were not treated with anticoagulation (16). In another large multinational study with 396 patients from birth to age 18 years, only 6 of 22 children (27%) with recurrent thrombosis (systemic or cerebral) were receiving anticoagulation at the time of recurrence, a finding that suggests that anticoagulation might prevent recurrent thrombotic events in the setting of pediatric CVST (9).
Two practice guidelines for pediatric CVST have been published. The AHA guidelines for the management of pediatric stroke state that anticoagulation in children with CVST with either unfractionated heparin or LMWH, even in the presence of hemorrhage, is reasonable (50). The American College of Chest Physicians (CHEST) guidelines recommend that children with CVST without hemorrhage be anticoagulated with either unfractionated heparin or LMWH. For children with coexisting hemorrhage, the CHEST guidelines state that anticoagulation may be administered. Alternatively, the patient can be followed with neuroimaging at 5 to 7 days with anticoagulation started if there is evidence of thrombus progression (52).
Both unfractionated heparin and LMWH have been used to treat pediatric CVST (1,9,16,21,23). Oral vitamin K antagonists such as warfarin have also been used (1), although less frequently since vitamin K antagonists usually take several days to become therapeutic. In general, if unfractionated heparin is used, a bolus or loading dose is generally avoided to prevent supratherapeutic treatment which may increase the risk of intracranial hemorrhage in the setting of acute neurologic injury. However, a hematologist should be consulted to provide individualized recommendations. Some centers have set a goal for the aPTT of 60 to 85 seconds (16), but the goal aPTT for each patient should be considered individually and may change during the course of treatment. An advantage of unfractionated heparin is the ability to completely reverse the medication in the case of major bleeding or for a surgical procedure. In recent years, LMWH has been utilized with increasing frequency (16). LMWH has several advantages over unfractionated heparin. Pharmacokinetics are predictable with LMWH, monitoring is required less frequently, heparin-induced thrombocytopenia is rare, and administration does not require intravenous access. Disadvantages of LMWH include twice daily injections and less easily accomplished reversal of anticoagulation. The goal anti-Xa level for a child on LMWH is often considered 0.5 to 1.0 units/mL 4 to 6 hours after a dose (16,50), but the goal anti-Xa level may be individualized. Dosing for both unfractionated heparin and LMWH is dependent upon both age and weight, and is often guided by a pediatric hematologist. Monitoring should ensure adequate anticoagulation without supratherapeutic values. It is important to remember that no clinical trial comparing unfractionated heparin to LMWH in the setting of CVST exists (50). A child on anticoagulation with any acute neurologic alteration or any worrisome feature such as headache or new seizures should be imaged with a noncontrast HCT immediately to assess for intracranial hemorrhage. Surveillance imaging for hemorrhage is not usually required without new or concerning neurologic symptoms.
Some children with CVST may be postoperative from mastoidectomy or otolaryngology procedures, craniosynostosis repair, or other surgeries at the time of CVST presentation. The decision to use anticoagulation must be multidisciplinary in the postoperative scenario and sometimes is started cautiously at a lower dose or after several days.
While no studies evaluating the duration of anticoagulation have been performed in pediatric CVST, most treat for 3 to 6 months (50,52). At hospital discharge, children are often transitioned from unfractionated heparin or LMWH to oral warfarin with a goal international normalized ratio of 2.0 to 3.0 (16,21). A new oral direct thrombin inhibitor that does not require PT monitoring, dabigatran, has been introduced in the United States but has not been studied in pediatric CVST.
Thrombolysis and Thrombectomy
The first case of local urokinase used to treat SSS thrombosis was reported by J.A. Scott et al in 1988 (56). The literature on thrombolysis and thrombectomy is limited to observational studies in the setting of CVST (57,58). The subjects of these studies were assumed to have poor prognosis and were comatose or had continued deterioration despite systemic anticoagulation. Mechanical thrombectomy was conceived as a method of obtaining rapid recanalization without the use of local thrombolytics to reduce the risk of hemorrhage. Nevertheless, most reported cases of thrombectomy were also treated with chemical thrombolysis (57).
A review of the literature from 1988 through June 2009 described 161 adult CVST patients who underwent direct chemical thrombolysis and 34 patients who underwent a combination of chemical and mechanical thrombolysis/thrombectomy. Overall, 87% of the 161 subjects treated with direct thrombolysis had excellent or good outcomes, and 35% of the 34 patients treated with chemical and mechanical thrombolysis/thrombectomy achieved near-complete recovery (59). The largest retrospective study comparing local thrombolysis and systemic anticoagulation included 20 adults treated with local urokinase and 20 adults treated with systemic heparin. The patients who received thrombolysis had better outcomes at discharge, but two patients had hemorrhagic complications. The authors concluded that additional randomized studies are necessary (58). The Cochrane Collaboration reviewed thrombolysis for CVST and concluded that without data from randomized controlled trials there is insufficient information to recommend thrombolysis routinely; however, the group also recognized that thrombolysis is being used more frequently and that a randomized controlled trial for its use in the acute phase is warranted (60). Pediatric literature on thrombolysis is limited to case reports. In one report of a child with extensive thrombosis and early signs of herniation, there was improvement of anterograde venous flow and clinical symptoms (61). In another series of three children treated with local thrombolysis, there was one death (22).
Although mechanical thrombectomy has mostly been reserved for patients with poor mental status, coma, straight sinus thrombosis, or a large space-occupying lesion, 60% of patients in one series had minimal or no deficits. However, 10% had moderate or severe neurologic deficits, and 30% died. The deaths were mostly due to intracranial hemorrhage and resulting herniation (57).
The AHA guidelines state that thrombolysis may be used in certain pediatric patients (50), and the CHEST guidelines state that thrombolysis/thrombectomy may be used in children who do not respond to anticoagulation (52). Nevertheless, the optimal subject, thrombolytic, dosage of thrombolytic, and mechanical device approach are not known. Furthermore, thrombolysis may not be successful in recanalyzing cortical veins.
Factor Replacement
Endogenous anticoagulant factor replacement with fresh frozen plasma can be considered in the setting of liver failure, nephrotic syndrome, or acquired deficiencies of antithrombin III, protein C, or protein S. However, no studies have demonstrated the efficacy of fresh frozen plasma in the setting of CVST.
Other Treatment Considerations
Children with iron deficiency anemia may require iron replacement or even transfusion of packed red blood cells if the anemia is severe. Children with sickle cell disease may require exchange transfusion or simple transfusion. In all cases of CVST in which an anemia is a risk factor, a hematologist should be consulted. In the case of young women on oral contraceptive pills, the medication should be discontinued immediately.
■ OUTCOME
Thrombus Propagation
One study of 79 children with CVST defined thrombus propagation as new thrombus in a venous sinus distal to or adjoining the initial thrombus within 14 days of the original diagnosis (16). Follow-up imaging was obtained within 14 days in 63 (80%) and showed clot propagation in 7 of 19 (37%) who were not treated with anticoagulation and in 3 of 44 (7%) children treated with anticoagulation (relative risk 3.1, 95% CI 1.6–5.8, P = .006). Propagation was symptomatic in 60% of the children and was accompanied by new venous infarction in 40%. Worse outcomes were associated with thrombus propagation (odds ratio 4.3, 95% CI 1.0–19.4, P = .053) (16).
Recanalization
The AHA guidelines state that repeated neuroimaging to assess for recanalization of the affected sinus(es) is sensible; however, the optimal timing of repeat imaging is not known. Many centers obtain follow-up imaging at 3 to 6 months when discontinuation of anticoagulation is typically considered (50). In mixed cohorts of neonates and children, the rates of recanalization are similar to those reported in adult series, with complete recanalization in 42% to 70%, partial recanalization in 23% to 42%, and persistent occlusion in 1% to 16%. (2,4,9,11,21,62). The presence of a prothrombotic risk factor was predictive of failure to recanalize in one series (16) but was not associated with failure to recanalize in another study (4). Longer duration of symptoms has been associated with failure to recanalize (11,21). The administration of anticoagulation has not been found to be significantly associated with recanalization (4,11,16). Furthermore, propagation of thrombus within 2 weeks has not been associated with recanalization at 3 months. Both a pediatric and an adult series found that neither the number of vessels involved nor deep sinus thrombosis predicted recanalization (11,62). In a retrospective study of 16 children with CVST, 10 children had follow-up imaging. Neurologic deficits were present in all three subjects without recanalization compared to in 29% of children with complete or partial recanalization (2). In another retrospective series of 53 neonates and children, there was no significant correlation between recanalization and neurologic outcome (21).
Recurrence
The recurrence rate of CVST in pediatric cohort studies has been reported between 3% and 8%. The recurrence rate of systemic thrombosis after CVST has been reported between 3% and 7% (1,9,11,23). These estimates are similar to those in an adult study (24). From a multicenter mixed cohort of children and neonates from Europe and Israel with CVST, there were 22 recurrent venous thromboses of which 13 (59%) were cerebral. The recurrence rate for any venous thrombosis was 21.1 per 1000 person-years (95% CI 13.9–32.1 per 1000 person-years). Recurrent CVST was not seen in children < 2 years in this cohort, and the recurrence rate for any venous thrombosis in children older than 2 years was 29.1 per 1000 person-years (95% CI 18.9–44.7 person-years). Most recurrent CVST occurred within the first 6 months from initial diagnosis. Lack of treatment with anticoagulation, failure to recanalize, and heterozygosity for the G20210A mutation were all significantly associated with an increased risk of any recurrent venous thrombosis in a Cox proportional hazards model (9). Other smaller series report recurrences in children with underlying medical conditions such as nephrotic syndrome (11).
Neurologic Outcome
Neurologic outcomes in children with CVST range from normal to death. Normal follow-up examinations have been reported in 26% to 67% (1,11,23). Mortality estimates range from 3% to 13% (9,11,23). Death can be from the CVST itself or from underlying conditions. Neurologic deficits are present in 38% to 62% (1,11). In one cohort, deficits were motor in 80%, cognitive in 10%, developmental in 9%, speech in 6%, and visual in 6% (1). In a recent cohort, despite normal total scores on the Wechsler Intelligence Scale for Children, 14 of the 21 children given the scale had a cognitive abnormality in at least one subtest (3). In two other cohorts, 17% and 32% of children who survived had headache or other signs of elevated ICP (7,11). Epilepsy has been reported in 5% to 25% (7,11,63).
Since most cohorts are relatively small, predictors of neurologic outcome have not been elucidated fully. In one cohort, mortality was associated with coma at presentation and with seizures (22). In another study, younger children, seizures, and involvement of the straight sinus were predictors of severe sequelae while any combination of focal neurologic findings, seizures, and decreased consciousness were associated with any neurologic sequelae (21). In the cohort from the Canadian Pediatric Ischemic Stroke registry, seizures in nonneonates and infarction in neonates and nonneonates were associated with the composite outcome of neurologic deficits or death (1). Infarction has been associated with disability (3,18). In one study, good cognitive outcome was associated with older age at CVST presentation, involvement of the lateral and/or sigmoid sinuses, and anticoagulation (11). Another cohort demonstrated similar predictors of outcome in univariate analysis, although the findings were not significant in multivariate analysis (3). Other reported predictors of an unfavorable outcome are absence of recanalization or partial recanalization, thrombus propagation, presence of intraventricular hemorrhage, involvement of the straight sinus, and neurologic comorbidity (16).
■ CONCLUSIONS
CVST is an important cause of morbidity and mortality in children. The presenting symptoms and clinical course are variable. When there is suspicion for CVST, dedicated cerebral vascular studies, including MRI/MRV or CTV, should be performed. Treatment decisions often rely on a multidisciplinary approach among intensivists, neurologists, hematologists, and occasionally neurosurgeons and interventional radiologists. CVST management includes supportive measures, identification and management of underlying risk factors, and in most cases anticoagulation. Systemic anticoagulation is generally safe, even in the presence of intracranial hemorrhage and may improve outcome, but further study is needed. Monitoring children with CVST demands close observation for neurologic deterioration. In the absence of randomized clinical trials, recommendations are based on data from cohort studies and from adult literature. The efficacy of any treatment, including anticoagulation, for the prevention of death or long-term neurologic sequelae requires adequately powered randomized controlled trials.
■ REFERENCES
1. et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–423.
2. et al. Cerebral sinus venous thrombosis in children. J Paediatr Child Health. 2004;40:53–55.
3. et al. Cerebral sinus venous thrombosis in Swiss children. Dev Med Child Neurol. 2010;52:1145–1150.
4. et al. Cerebral venous thrombosis in children: a multifactorial origin. Circulation. 2003;108:1362–1367.
5. et al. Occurrence of cerebral venous sinus thrombosis in patients with presumed idiopathic intracranial hypertension. Ophthalmology. 2006;113:2281–2284.
6. , . Features of dural sinus thrombosis simulating pseudotumor cerebri. Eur J Neurol. 1999;6:601–604.
7. et al. Long-term prognosis of cerebral venous sinus thrombosis in childhood. Dev Med Child Neurol. 2004;46:514–519.
8. . Developmental hemostasis: relevance to thromboembolic complications in pediatric patients. Thromb Haemost. 1995;74:415–425.
9. et al. Risk factors for recurrent venous thromboembolism in the European Collaborative Paediatric Database on Cerebral Venous Thrombosis: a multicentre cohort study. Lancet Neurol. 2007;6:595–603.
10. et al. Cerebral venous thrombosis in children. J Child Neurol. 2001;16:574–580.
11. et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477–489.
12. et al. 2D time-of-flight MR venography in neonates: anatomy and pitfalls. AJNR Am J Neuroradiol. 2006;27:1913–1918.
13. et al. MR venography in the pediatric patient. AJNR Am J Neuroradiol. 2005;26:50–55.
14. et al. Development of posterior fossa dural sinuses, emissary veins, and jugular bulb: morphological and radiologic study. AJNR Am J Neuroradiol. 1994;15:1871–1883.
15. et al. Lateral sinus thrombosis as a complication of otitis media: 10-year experience at the children’s hospital of Philadelphia. Pediatrics. 2009;123:709–713.
16. et al. Anticoagulants in pediatric cerebral sinovenous thrombosis: a safety and outcome study. Ann Neurol. 2010;67:590–599.
17. et al. Arterial ischemic stroke and cerebral venous thrombosis in children: a 12-year Argentinean registry. Acta Haematol. 2006;115:180–185.
18. et al. Cerebral sinovenous thrombosis in the neonate. Arch Neurol. 2006;63:405–409.
19. et al. Cerebral venous sinus thrombosis in infancy and childhood: role of genetic and acquired risk factors of thrombophilia. Eur J Pediatr. 1998;157:555–560.
20. et al. Neonatal cerebral sinovenous thrombosis from symptom to outcome. Stroke. 2010;41:1382–1388.
21. et al. Cerebral sinovenous thrombosis in children: clinical presentation and extension, localization and recanalization of thrombosis. Eur J Paediatr Neurol. 2010;14:80–85.
22. et al. Cerebral venous sinus thrombosis in children: a multicenter cohort from the United States. J Child Neurol. 2008;23:26–31.
23. et al. Anticoagulation therapy in pediatric patients with sinovenous thrombosis: a cohort study. Arch Neurol. 1998;55:1533–1537.
24. et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664–670.
25. et al. Cerebral venous sinus thrombosis: a case series including thrombolysis. Arch Dis Child. 2009;94:790–794.
26. et al. Cerebral venous thrombosis in neonates and children. Pediatr Neurol. 1992;8:112–116.
27. et al. Cerebral venous thrombosis in childhood. Eur Radiol. 2001;11:1760–1765.
28. et al. Neonatal dural sinus thrombosis. Pediatr Neurol. 1989;5:161–165.
29. et al. Cerebral sinovenous thrombosis after closed head injury. J Trauma. 2009;66:1599–1604.
30. et al. Superior sagittal sinus thrombosis associated with Evans’ syndrome of haemolytic anaemia. J Neurol. 1985;232:280–282.
31. et al. Cerebrovascular complications of l-asparaginase in the therapy of acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2003;25:484–487.
32. et al. Sequelae of thrombotic or hemorrhagic complications following l-asparaginase therapy for childhood lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1988;10:191–195.
33. et al. Hemostatic problems and thromboembolic complications in nephrotic children. Pediatr Nephrol. 2000;14:138–142.
34. , , . Cerebral sinovenous thrombosis and idiopathic nephrotic syndrome in childhood: report of four new cases and review of the literature. Eur J Pediatr. 2006;165:709–716.
35. et al. Cerebral vein thrombosis in childhood systemic lupus erythematosus. J Pediatr. 1995;126:722–727.
36. et al. The long-term mortality and morbidity of Behcet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore). 2003;82:60–76.
37. et al. Pediatric Behçet’s disease and thromboses. J Rheumatol. 2010;38:387–390.
38. et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539–1543.
39. et al. Prothrombotic risk factors in childhood stroke and venous thrombosis. Eur J Pediatr. 1999;158(suppl 3):S117–S121.
40. et al. Paediatric cerebral sinus vein thrombosis. A multi-center, case-controlled study. Thromb Haemost. 2004;92:713–718.
41. et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation. 2010;121:1838–1847.
42. , , . Elevated plasma factor VIII and D-dimer levels as predictors of poor outcomes of thrombosis in children. N Engl J Med. 2004;351:1081–1088.
43. et al. Familial elevated factor VIII in children with symptomatic venous thrombosis and post-thrombotic syndrome: results of a multicenter study. Arterioscler Thromb Vasc Biol. 2006;26:1901–1906.
44. , . Magnetic resonance imaging of patients with epilepsy. Clin Radiol. 2001;56:787–801.
45. et al. Cerebral veins: comparative study of CT venography with intraarterial digital subtraction angiography. AJNR Am J Neuroradiol. 1999;20:249–255.
46. et al. Interobserver variability in the detection of cerebral venous thrombosis using CT venography with matched mask bone elimination. Clin Neurol Neurosurg. 2009;111:717–723.
47. et al. Diagnostic value of multidetector-row CT angiography in the evaluation of thrombosis of the cerebral venous sinuses. AJNR Am J Neuroradiol. 2007;28:946–952.
48. et al. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169:1699–1707.
49. et al. Comparison of CT venography with MR venography in cerebral sinovenous thrombosis. AJR Am J Roentgenol. 2006;187:1637–1643.
50. et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008 39:2644–2691.
51. et al. Emergent decompressive craniectomy in patients with fixed dilated pupils due to cerebral venous and dural sinus thrombosis: report of three cases. Neurosurgery. 1999;45:626–629; discussion 629–630.
52. et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (9th Edition). Chest. 2012;141(2 Suppl):e737S–801S.
53. et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338:597–600.
54. , . Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30:484–488.
55. , , . Anticoagulation for cerebral sinus thrombosis. Cochrane Database Syst Rev. 2002:; CD002005 .
56. et al. Treatment of dural sinus thrombosis with local urokinase infusion. Case report. J Neurosurg. 1988;68:284–287.
57. et al. Endovascular thrombectomy and thrombolysis for severe cerebral sinus thrombosis: a prospective study. Stroke. 2008;39:1487–1490.
58. et al. Nonrandomized comparison of local urokinase thrombolysis versus systemic heparin anticoagulation for superior sagittal sinus thrombosis. Stroke. 2001;32:2310–2317.
59. et al. Direct thrombolysis for cerebral venous sinus thrombosis. Neurosurg Focus. 2009;27:E7 .
60. et al. Thrombolysis for cerebral vein and dural sinus thrombosis. Cochrane Database Syst Rev. 2004; CD003693 .
61. et al. Local fibrinolysis in cerebral venous thrombosis. Pediatr Neurol. 1994;10:78–80.
62. et al. Cerebral venous thrombosis: correlation between recanalization and clinical outcome—a long-term follow-up of 40 patients. J Neurol. 2002;249:1123–1124.
63. et al. Short-term intellectual outcome after arterial ischemic stroke and sinovenous thrombosis in childhood and infancy. J Child Neurol. 2005;20:553–559.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


