Considerations in Routine Prenatal Care
• OB review of systems: Every encounter ask about VB, LOF, CTX, & FM, & other systems by complaint.
1st FM: 16–18 w if multiparous, 18–20 w if nulliparous
• Physical: BP, weight (current & interval change), FHR, & FH at each visit. Complete PE & pelvic exam at 1st prenatal visit.
FHR: Detected by Doppler at 10–12 w & by fetoscope at 18–20 w (w/ nml BMI)
• Cervical exam: Assess dilation, effacement, station near term.
• Psychosocial screening: Tobacco use, EtOH use, DV, nutrition, psychosocial situations, job-related risks & high-risk behaviors.
Tobacco: Encourage tobacco cessation each visit; ∼50% of 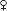 quit smoking during or before their Preg. ∼50% resume smoking w/i 1-y postpartum. A/w IUGR, low birth weight, placental abruption, placenta previa, PPROM, ectopic Preg, & perinatal mortality. Children of smokers ↑ asthma, colic, obesity, & SIDS. Counsel using 5 A’s strategy (Ask, Advise, Assess, Assist, Arrange). Nicotine replacement not well assessed, but likely safer than smoking. Bupropion & varenicline less used in Preg.
quit smoking during or before their Preg. ∼50% resume smoking w/i 1-y postpartum. A/w IUGR, low birth weight, placental abruption, placenta previa, PPROM, ectopic Preg, & perinatal mortality. Children of smokers ↑ asthma, colic, obesity, & SIDS. Counsel using 5 A’s strategy (Ask, Advise, Assess, Assist, Arrange). Nicotine replacement not well assessed, but likely safer than smoking. Bupropion & varenicline less used in Preg.
EtOH: No safe threshold a/w mental retardation, neurologic deficits, fetal EtOH syn (esp w/ chronic EtOH use; growth restriction, facial anomalies, & CNS deficits).
DV: Red flags include unwanted Preg, late presentation for PNC, substance abuse, poor weight gain, & multi somatic complaints.
• GDM screening: 2-step approach w/ GLT then GTT. See Chap. 17. Perform btw 24 & 28 w. Opt out for extremely low risk considered (age <25, BMI <25, no FHx of DM, no personal h/o gluc intolerance, no h/o adverse obstetrical outcomes a/w DM, & not of an ethnic group w/ ↑ risk DM).
• Vaccines: See Chap. 1. Influenza vaccine recommended for all pregnant women. TDaP recommended for all in 3rd trimester (↑ transplacental IgG immunity for neonate) or postpartum if >10 y since last dose. (MMWR 2011;60:1424). Postpartum vax for rubella or varicella if nonimmune.
• GBS screening at 35–37 w or if deliv anticipated (every Preg) (Obstet Gynecol 2011;117:1019). See Chap. 10. Swab lower vagina, introitus, & rectum. Cx valid for 5 w. For pts w/ sev PCN allergy (anaphylaxis, angioedema, respiratory distress, urticarial) → request clindamycin & erythromycin sens testing.
Physiologic Changes of Pregnancy (Best Pract Res Clin Obstet Gynaecol 2008;(5):801)
• Cardiovascular: ↓ SVR → ↑ HR. BP ↓ early (∼10% by 7–8 w) → nadir at 24 w → gradual ↑ to term. Cardiac output ↑ in 1st trimester → peaks in 2nd trimester at 30–50% above nonpregnant values. See Chap. 12.
• Respiratory: O2 consump ↑ 30–50 mL/min (2/3 due to mat requirement, 1/3 for fetal). Tidal vol ↑ to 500–700 mL (prepregnancy of 200 mL). Respiratory rate unchanged. Minute ventilation ↑ from 7.5–10.5 L/min. Functional residual capacity ↓ by 500 mL. Vital capacity unchanged. See Chap. 13.
• Renal: Renal bld flow ↑ 35–60%. Kidneys ∼1 cm larger w/ ↑ in bld vol; renal pelves, calyces, & ureters ↑ in size in resp to progesterone. GFR ↑ 40–50%, peaks at 180 mL/min by the end of 1st trimester. See Chap. 14.
• Gastrointestinal: Progesterone → ↓ esoph sphincter tone → GERD. Delayed gastric emptying & ↑ intestinal transition time. Increased constip. See Chap. 15.
• Hematologic: Plasma vol ↑s rapidly. 10% ↑ by 7 w → plateau at 32 w ∼50% above nonpregnant → dilutional anemia of Preg. Red cell mass ↑ 18–25% secondary to ↑ erythropoietin. Nml Preg Hgb 11–12 g/dL. WBC ↑ in 1st trimester → plateau at 30 w. Nml Preg WBC 5000–12000/mm3. Platelet count ↓ due to dilution &/or increased consump. Mild thrombocytopenia (100000–150000/mm3) seen in ∼8% of pregnancies. Preg is a procoagulable state, predisposing to thromboembolisms w/ 4–6 fold ↑ DVT. Factors VII, VIII, IX, X, & XII; fibrinogen; von Willebrand factor; antithrombin III; & prot C ↑. Factor XI & prot S ↓. Prothrombin & Factor V are unchanged. See Chap. 16.
• Endocrine: ↑ hepatic production of thyroid-binding globulin → ↑ total T4. Free T4 essentially unchanged (except for transient ↑ from hCG’s thyrotropin-like activity in 1st trimester). TSH falls in 1st trimester, then normalizes. No real change in mat thyroid status. Pancr islet cells undergo hyperplasia → ↑ insulin secretion. Placental factors ↓ mat insulin sens. Pituitary ↑ 135%, but no optic nerve compression. Prolactin levels peak at term. See Chap. 17.
NUTRITION IN PREGNANCY
Weight Management
• Caloric intake: Encourage balanced diet.
1st trimester: No additional caloric intake from baseline
2nd trimester: ↑ 340 kcal/d from baseline
3rd trimester: ↑ 452 kcal/d from baseline
• Obesity in Preg: ↑ complications w/ ↑ BMI. Encourage preconception weight loss. Preg is high-risk period for excessive weight gain → long-term obesity. Nutrition consultation: Encourage adherence to 0.45–9.1 kg (11–20 lb) weight gain. Pregnant women w/ BMI >30: ↑ rates of GHTN, preeclampsia, gestational diabetes, macrosomia, & cesarean deliv. Consider HbA1C or early GLT for pre-existing diabetes.
• Exercise in Preg: ACOG recommends ≥30 min of mod daily exercise. Avoid activities w/ high risk for abdominal trauma (eg, horseback riding, skiing/snowboarding), or Scuba diving. Terminate exercise w/ bleeding, preterm labor, ↓ FM, LOF, chest pain, dizziness, dyspnea prior to exertion. Absolute contraindications to exercise: Heart or lung dzs, incompetent cervix, multi gest, VB, placenta previa, pregnancy-induced HTN, rupture of membranes (Int J Gynaecol Obstet 2002;77:79).
Food Warnings
• Methylmercury: High levels can cause CNS damage & mild dysfxn in fetus. Avoid: Shark, swordfish, king mackerel, or tilefish. Limit albacore tuna to 6 oz/w. Encourage 12 oz (∼2 servings) of low mercury fish weekly.
• Caffeine: Mod consump safe (<200 mg/d). One 8 oz coffee = ∼95 mg caffeine. Mod (<200 mg/d) consump not a/w miscarriage (Am J Obstet Gynecol 2008;198:279). No clear evid for caffeine ↑ risk of IUGR (JAMA 1993;269:593).
• Vit A: Limit to 750 μg/d (Lancet 2010;375:1640). Deficiency common in developing countries. Supplements improve night blindness & anemia w/o teratogenicity. >3000 μg/d (10000 IU) → ↑ fetal malformations.
• Food-borne illness: Encourage good hand hygiene & thorough cooking
Listeriosis: Processed meats, soft cheeses, meat spreads, & pate.
Brucellosis: Unpasteurized milk & cheese made from raw milk.
Toxoplasmosis: Undercooked meats & contaminated vegetables > cat feces.
• Pica: Consuming nonfood substances (J Am Diet Assoc 1991;91:34). More common in Preg. Avoid pica & screen for iron-deficiency anemia (unclear mech). Can → lead tox or infectious dz (esp developing settings).
Nutrients in Pregnancy
• Folic acid: ↓s NTDs. NT forms during week 4 of gest → start folate prior to Preg. Low-risk women, use 0.4 mg/d (common dose in prenatal vitamins). Women w/ h/o NTD in prior Preg → 4 mg/d (72% ↓ in recurrence risk). If on antiepileptic drugs, also ↑ folate dose.
• Vit D: Deficiency common in Preg (newborn levels dependent on mat levels), esp vegetarians, limited sun exposure, & dark-skinned ethnicity. Deficiency = 25-OH-D < 20. No routine screening for Vit D in Preg. Suppl w/ 1000–2000 IU/d (Obstet Gynecol 2011;118:197).
CLINICAL PELVIMETRY
Pelvic Anatomy
• Pelvis: Sacrum, coccyx, & innomin bones. Innomin = ilium, ischium, & pubis → join sacrum at sacroiliac jnts & each other at symphysis pubis.
• Linea terminalis (aka innomin line): Divides false & true pelves
False pelvis: Above linea terminalis, bounded by lumbar vertebra, iliac fossa, & anter abdominal wall
True pelvis: Clinically important for parturition; it includes:
Post: Anter surface of the sacrum
Lateral: Inner surface of ischial bones
Anter: Pubic bones & ascending rami of ischial bones
Planes and Diameters of the Pelvis
• Obstetric Conjugate (OC; aka AP diameter): Obstetrically relevant diameter. Shortest distance btw the promontory of the sacrum & the symphysis pubis. Measured indirectly by subtracting 1.5–2 cm from the diagonal conjugate.
• Diagonal conjugate: Distance btw lower margin of symphysis to sacral promontory. Measured clinically w/ examining hand & used to calculate OC.
• Transverse diameter: Distance btw linea terminalis on either side. At right angle to obstetrical conjugate. Largest diameter of pelvis.
• Interspinous diameter: In midpelvis. Smallest pelvic diameter, but usually >10 cm.
Figure 9.1 Pelvic shapes
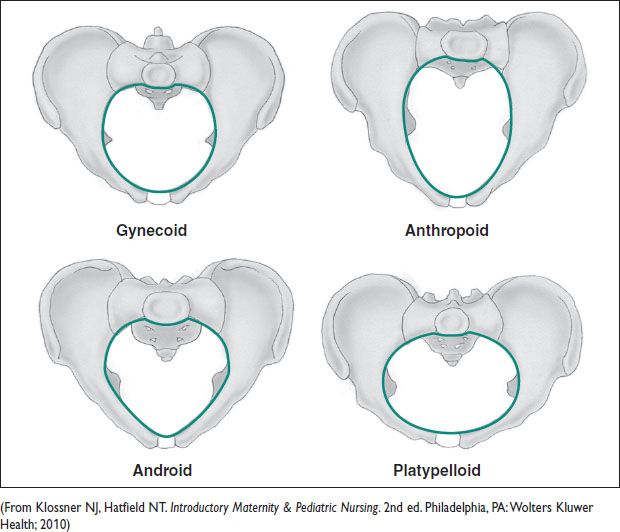
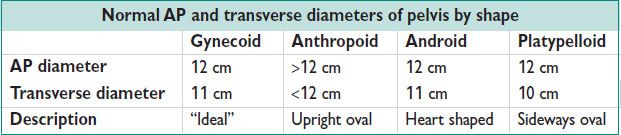
Pelvic Shapes
• Caldwell & Moloy classification: Describes 4 ideal types, recognizing there are variations in pelvic shape. Characterized primarily by the transverse & interspinous diameter.
• Gynecoid: Deemed “ideal” w/ wide pelvic inlet & outlet & straight sidewalls
• Anthropoid: Narrow transverse diameter but wide AP diameter
• Platypelloid: Wide inlet & outlet w/ narrow AP diameter & sacral inclination
• Android: Straight sidewalls w/ narrow subpubic arch & narrow incline of sacrum
Pelvimetry in OB Practice
• Clinical: Clinical exam of pelvis to predict CPD
Clinical pelvimetry = poor predictor of CPD
• Radiologic pelvimetry: X-ray or MRI to predict CPD. Radiographic pelvimetry studies → no impact on mat or neonat morbidity or mortality (Cochrane Database Syst Rev 2000:CD000161).
• Pelvimetry largely replaced by trial of labor. No evid to recommend Cesarean deliv for concerns for CPD based on clinical or radiographic pelvimetry.
COMMON PRENATAL COMPLAINTS
Nausea and Vomiting (Obstet Gynecol 2004;103:803)
• NVP: 70–85% of pregnancies. ↑ hCG & estrogen → NVP. Typically presents <9 w ± abdominal pain. If abd pain & fever → broader diff. 50% resolves by 14 w; 90% by 22 w (Am J Obstet Gyn 2000;182:931).
Therapy: Small, frequent meals w/ bland low-fat foods (BRAT diet). Use of ginger can be effective. Encourage hydration.
1st-line meds: Vit B6 (10–15 mg TID-QID) & antihistamines (doxylamine)
2nd-line meds: Promethazine, metoclopramide, then ondansetron
• Hyperemesis Gravidarum (HEG): NVP significant enough to cause dehyd, metabolic alkalosis, ketonuria, weight loss (>5%), hypokalemia. <1% of pregnancies. Risks: Multi gest, FHx, or personal Hx in prior Preg.
• W/u: Labs may show elevated transaminases (<300), Amy, & lipase; hypochloremic metabolic alkalosis; suppressed TSH & ↑ thyroxine; ketones on UA
• Therapy: IV hydration (w/ dextrose ± thiamine), enteral nutrition (eg, tube feeding), hospitalization for monitoring & suppl as above
Carpal Tunnel Syndrome (CTS) (Muscle Nerve 2006; 34:559)
• Incid btw 2 & 35%; most often in 3rd trimester. Risks: H/o CTS in prior Preg, age >30, nulliparous, edema. Caused by compression of median nerve related to edema in Preg. Sx include numbness, pain, paresthesias of thumb, index, & middle fingers, often worse at night. Exacerbated by flexion or extension of wrist, improved by mvmt of hands.
• Exam: ± median nerve sensory deficit. Phalen test: Pain reproduced w/ prolonged (>60 s) flexion of wrists. Tinel test: Pain reproducible w/ percussion at wrist over median nerve.
• Rx: Low salt diet, physical therapy, wrist bracing, Tylenol → consider Cort injections for refrac cases. Surgical intervention generally not indicated, sx improve w/i 1 y of deliv (4–50% persist at 1 y).
Round Ligament Pain
• Anatomy: Origin at uterine fundus → inguinal canal, terminates in labia majora.
• Presentation: Lower abdominal pain (more common in right lower quadrant). Exacerbated by mvmt, often reported as “shooting pain into vagina.” Case reports of association w/ endometriosis, lipomas, & varicosities. Dx depends on ruling out other etiologies (eg, torsion, appendicitis, preterm labor).
• Rx: Typically self-limited. Advise acetaminophen, rest, & reassurance. Belly-band can be helpful.
Lower Extremity Edema
• Physiologic changes in Preg predispose to edema dev. SVR ↓, venous return impeded by gravid uterus. Water retention mediated by ↓ plasma osmolality due to osmolar reset of vasopressin & thirst thresholds (Br J Obstet Gynaecol 1985;92:1131).
• Rx: Elevation of feet & support stockings. Counsel women to report nonsymmetric edema or nondependent edema as these can be signs of pathology such as DVT or preeclampsia.
Low Back Pain (Obstet Gynecol 2004;104:65)
• Up to 70% report LBP during Preg. Risks: LBP outside of Preg, in a prev Preg, or w/ menstruation.
• Presentation: Attributed to changes in posture & joint laxity. Pain exacerbated by mvmt, relieved by rest. ± assoc neurologic sx.
• Exam: Eval motor/sensory fxn & reflexes to detect radiculopathy. Paraspinal or joint tenderness to palpation & ↓ range of motion. Imaging not indicated in the absence of progressive neuro signs or trauma.
• Rx: Avoid excessive weight gain, lifting heavy objects, prolonged standing, bending from waist. Recommend shoes w/ arch support & sleeping on side w/ pillow btw knees. Use of good body mechanics when lifting & getting out of vehicles is critical. Exercise, acupuncture, support belts may be helpful adjuncts (Cochrane Database Syst Rev 2007;18(2)).
Lower Extremity Varicosities
• Pathophysiology: Femoral venous pres ↑ in Preg up to 24 mm Hg secondary to uterine compression on IVC. Pressures closer to 8 mm Hg (pregravid state) in lateral recumbent position (Surg Gynecol Obstet 1950;90:481).
• Presentation: Sx vary from cosmetic complaints to a range of discomfort. Throbbing pain that may worsen w/ advancing Preg, weight gain, & standing.
• Rx: Periodic elevation of feet & support stockings. Surgical correction during Preg generally avoided unless sev sx.
Vulvar Varicosities
• Pathophysiology: 4% lifetime prevalence, most often occurring during Preg b/c of ↑ venous pressures & ↑ pelvic bld flow. “Vulvar veins lack valves”
• Presentation: Often asymptomatic & noted only on exam. Pelvic discomfort & swelling worsened with standing or intercourse.
• Mgmt: Reassurance – most vulvar varicosities regress postpartum. Vulvar support belt for sev sx or local excision for thrombosis. Vaginal deliv not contraindicated despite theoretical risk of hemorrhage w/ laceration.
Hemorrhoids
• Pathophysiology: Arise w/i plexus of inferior & superior hemorrhoidal veins. ↑ venous pressures in Preg → engorgement both internally & externally → venous stasis → thrombosis & pain/swelling.
• Presentation: Painless bleeding w/ defecation or anal pruritus. Sev pain or complaints of a palpable lump can occur w/ thrombosis. External hemorrhoids visualized as dilated veins; thrombosis felt on palpation during rectal exam.
• Rx: Supportive w/ local anesthesia, hydration, & stool softeners. Topical anesthetics or steroid creams along w/ warm soaks can provide local relief. Thrombosis can be treated w/ excision under local anesthesia.
FETAL ULTRASOUND: ANATOMY AND ECHOCARDIOGRAPHY
Basic Second Trimester Ultrasound (Obstet Gynecol 2009;113:451)
• Fetal viability: Fetal cardiac activity (including HR & any abn rhythms)
• Fetal number: In multi gestations, document chorionicity (number of placentas), amnionicity (number of membranes), fetal gender, comparison of fetal size, amniotic fluid.
• AFV: Described subjectively or by semiquantitative methods
AFI: Sum of depth (cm) of fluid pockets not containing cord or fetal extremities in each of 4 quadrants of the uterus. Quadrants divided by intersection of umbilicus & linea nigra.
SDP: Vertical depth (cm) of deepest pocket of fluid not containing cord or fetal extremities. Also called MVP, other.
• Placental location: Describing location (anter/post) & relation to internal os.
Endovaginal US should be performed if internal cervical os not clearly visualized.
Placental abnormalities (eg, previa) should be followed up w/ 3rd trimester US.
• Umbilical cord: The number of umbilical arteries should be noted.
• CL: Not currently a rec for low-risk pop. Recommendations for CL screening are evolving. Screening CL at anatomy US after 16 w GA is reasonable. Endovaginal US w/ empty bladder more accurate.
• GA: Most accurate in 1st trimester; 2nd trimester determination includes:
BPD: Measured at level of thalamus & cavum septum pellucidum.
HC: More reliable than BPD if head shape flattened or rounded.
AC: Measured at junction of umbilical vein, portal sinus, & stomach. Can compare to BPD to determine symmetric macrosomia or IUGR.
FL: Long axis of femur not including the distal & prox epiphyses.
• EFW: Combination of BPD, HC, AC, & FL to determine EFW.
EFW compared to known values to establish %ile & establish macrosomia/IUGR.
Fetal Anatomy Ultrasound (J Ultrasound Med 2010;29:157)
• Routinely performed at 18–20 w GA. Thorough assessment of fetal structures.
Head, face, neck: Cerebellum, choroid plexus, cisterna magna, lateral cerebral ventricles, midline falx, cavum septum pellucidum, upper lip
Chest: Cardiac exam including 4-chamber view & outflow tracts
Abd: Stomach, kidneys, bladder, umbilical cord insertion, umbilical cord vessels
Spine: Including cervical, thoracic, & sacral spine
Extremities: Arms & legs including feet & hands
• Routine screening:
RADIUS trial: 15151 women randomized to screening US vs. US only if other indications; detection rate of 34% vs. 11%, respectively, for fetal anomalies; no change in other outcomes (Am J Obstet Gynecol 1994;171:392).
Eurofetus trial: Large 3-y study revealing 61% sens of US to detect fetal anomalies. Neurologic/urologic anomalies more commonly detected than cardiac (88/89% vs. 20%) (Am J Obstet Gynecol 1999;181:446).
Detection depends on prevalence of anomalies. Detection of anomalies higher in academic compared to community centers. Image quality has improved tremendously since these trials. More anomalies can be identified, though the clinical implications are unclear.
• Aneuploidy screening: US alone not adequate for trisomy 21 (T21) or other aneuploidy. Presence or absence of fetal anomalies a/w T21, such as cardiac anomalies & duodenal atresia, confers ↑ or ↓ risk, respectively. ↑ NT on 1st trimester US identifies ↑ risk of aneuploidy. Soft markers: Echogenic bowel, EIF, short femur or humerus, & dilated renal pelvis. Absence of “soft markers” for Down syn on US ↓ a priori risk of T21 or mat serum screening risk by 50%.
Fetal Echocardiography (J Ultrasound Med 2011;30:127)
• CHD: Leading cause of mortality & morbidity. Prenatal dx offers planning for infant & intervention at birth.
• Indications: Used as adjunct to routine US screening, btw 18 & 22 w
• Mat indications: Autoimmune antibodies, familial inherited cardiac d/o, 1st- or 2nd-degree relative w/ CHD or syndromes w/ CHD, IVF, metabolic dz, cardiac teratogen exposure, rubella exposure 1st trimester
• Fetal indications: Abn cardiac screening exam, abn HR or rhythm, fetal chromosomal anomaly, extracardiac anomaly, hydrops, ↑ NT, monochorionic twins, unexplained sev polyhydramnios
CONGENITAL ANOMALIES
Definitions and Terminology
• Terminology: Description related to etiology
Malformation: Due to an intrinsic process in embryonic dev (prior to 8 w).
Deformation: Due to intrauterine process unrelated to fetus (eg, tumor, multi gest).
Disruption: Due to interference w/ nml dev (eg, amniotic band syn).
Dysplasia: Due to abn growth of cells into tissues.
• Patterns of anomalies: Multi anomalies can be described by overarching descriptors.
Syndrome: Assoc anomalies due to single pathologic etiology (eg, Turner syn)
Sequence: Group of anomalies related to a common upstream pathologic cause (eg, Potter’s sequence in which renal agenesis → oligohydramnios → bone fractures).
Developmental field defect: Due to disruption of dev in a particular region of the embryo that leads to disruption in related areas (eg, bladder exstrophy)
Association: Group of anomalies unrelated pathologically occurring more commonly than one would expect by chance (eg, VACTERL association).
Teratogens
• Definition: An agent that causes an anomaly in the developing fetus.
Mat illness: Due to toxic metabolites or antibodies from mother crossing placenta.
Pregestational diabetes: 6–7% risk (2× nml pop) of congen anomalies including NTDs, congenital heart disease (CHD) & caudal agenesis (rare but 15–20% causes a/w DM).
Systemic lupus erythematosus: A/w fetal congen complete heart block.
Infxn: Commonly TORCH infxns, varicella, or parvovirus B19. Nonspecific US findings: Microcephaly, calcifications, IUGR, HSM, hydrops, cardiac malformations.
Meds: Thalidomide & its association w/ limb reduction is classic example.
Environmental: Lead, ionizing radiation, fever, hyperthermia, & mercury consump.
Neural Tube Defects (Int J Gynaecol Obstet 2003;83:123)
• Epidemiology: 1.4–2 per 1000 pregnancies; 2nd most common anomaly worldwide.
• Etiology: NTDs not a/w syndromes can be genetic or environmental.
Genetic: Risk of NTDs higher in pts who have a child w/ prior NTD; only 5% of NTDs have familial association.
Environmental: Assoc factors include diet (low folic acid consump), teratogen exposure (anticonvulsants, Vit A), mat diabetes w/ poor 1st trimester gluc control, high mat core temperature in the 1st trimester.
• Pathophysiology: Failure of closure
Cranial defects: Egs, anencephaly, encephalocele, exencephaly, iniencephaly. All cranial defects except small encephaloceles (failure of skull formation w/ extrusion of brain into membranous sac) are lethal. Termination of Preg valid option.
Spinal defects: Often a/w ventriculomegaly (often require shunt placement)
Spina bifida: Failure of fusion of caudal portion of neural tube
Meningocele: Failure of fusion, meninges exposed
Meningomyelocele: Failure of fusion, meninges & neural tissue exposed
• Clinical manifestations: Higher lesions generally indicate worse prog
Bladder/bowel: Dysfxn common, even w/ lower spinal lesions. Bladder dysfxn → UTIs, stones, & significant morbidity. Sexual dysfxn common.
Neuro: Sensory & motor handicap correlated w/ level of lesion; ventriculomegaly a/w ↓ intelligence quotient.
• Dx: ↑ amniotic fluid & mat serum AFP
Screening: 89–100% of pregnancies w/ NTD have ↑ MSAFP
Other causes of ↑ MSFAP: (1) incorrect GA, (2) multi gestations, (3) abdominal wall defects, (4) abnormalities of placentation such as accreta (↑ MSAFP risk factor for placental abruption), (5) IUFD, (6) Finnish nephrosis, (7) sev skin anomalies such as lethal ichthyosis.
US able to identify many causes – done after MSAFP collection at a GA that will allow for detailed analysis of fetal anatomy.
US: 97% sens & 100% spec for NTD in experienced centers.
Dx: 2% of women w/ positive MSAFP have fetus w/ NTD. Confirmatory test can be an amniocentesis for AFP.
If ↑ amniotic AFP → confirmatory testing (AF acetylcholinesterase – 2.2/1000 false positive rate)
• Prevention: Avoidance of teratogens & suppl w/ folic acid (see Nutrition)
This behavior should start prior to Preg & continue throughout Preg.
• Rx: Deliv at hospital w/ NICU support; consideration of fetal Surg
Breech presentation common in fetus w/ NTD necessitating Cesarean deliv; vaginal deliv should be considered if fetus in cephalic presentation.
Other Neurologic Anomalies
• Ventriculomegaly: ↑ vol of cerebral ventricles on US.
Isolated: Often found to be a/w NTD or other malformations after birth.
Associations: Can be related to infxn (toxoplasmosis, CMV, lymphocytic choriomeningitis virus), genetic syndromes, or aneuploidy.
W/u: Amniocentesis should be offered for aneuploidy/infxn w/u. F/u 3rd trimester scan should look for progression or other identifiable causes.
• Hydrocephalus: Pathologic ventriculomegaly from ↑ pres
• CPCs: Cystic sonolucent lesions w/i choroid plexus
Not a true anomaly, but identified as marker of aneuploidy (esp Trisomy 18).
Isolated CPCs usually benign & typically resolve by 3rd trimester.
Cardiovascular Anomalies
• Nonimmune hydrops fetalis NIHF: Cardiac anomalies cause up to 40% of NIHF.
Manifestations: Pts can present w/ size > dates & ↓ FM. US: Ascites (visualized as rim of fluid around abdominal organs), pleural effusions, pericardial effusions, skin edema (late finding), polyhydramnios, & placentomegaly.
Associations: Structural heart dx, tachyarrhythmias (treated by giving rate-controlling agents to mother or directly to fetus), or bradyarrhythmias.
• Hypoplastic left heart syndrome HLHS:
Anatomy: Underdeveloped LV w/ hypoplasia, stenosis, or atresia of aortic valve, MV, &/or aorta. Survival dependent on PDA & ASD to allow for flow from RV to aorta.
Dx: Identified on US w/ findings of small or nonfunctioning LV, small aortic root, small aortic arch, ↑ or absent Doppler velocities through the aortic valve, abn MV, & restricted or reversed flow through the foramen ovale (usually right to left flow in utero).
Associations: Trisomy 18, trisomy 13, Turner syn, or sporadic
Mgmt: Identification can allow for birth planning (administration of prostaglandins to ensure persistent PDA) & poss fetal intervention. Dilation of AS can reverse HLHS physiology. In utero atrial septostomy can allow for ASD creation.
• AVSDs: Atrial & ventricular septal defects w/ singular, multileaflet atrioventricular valve. Diagnosed on US, confirmed w/ echo. AVSDs a/w aneuploidy.
• Conotruncal anomalies: Tetralogy of Fallot, persistent truncus arteriosus. Should prompt testing for DiGeorge syn (microdeletion of chromo 22q11, detectable by FISH).
Thoracic Anomalies
• CCAM:
Sporadic lesion due to abnormalities in branching of pulm tree → cystic or solid lung lesions. Classified based on size cystic or solid components. Different types confer varying risks of regression, progression, or malig transformation.
Type 1: Large (>2 cm) multiloculated cysts
Type 2: Smaller uniform cysts
Type 3: Not grossly cystic → “adenomatoid” type
Can lead to hydrops if large enough to cause mediastinal shift. Rx usually resxn at birth w/ peds at deliv.
• Congenital diaphragmatic hernia CDH: Defect in diaphragm → herniation
Diagnosed as solid (on right due to liver) or cystic (on left due to bowel) mass on US.
Occurs as isolated finding, as part of a sequence, or w/ aneuploidy (10–20%).
Left-sided lesions more common. Right-sided lesions confer worse prog (liver herniation). ↑ fetal lung vol improves prog. Can lead to NIHF & dextroposition.
Further w/u includes fetal echo, fetal karyotype, & poss MRI.
Gastrointestinal Anomalies
• Omphalocele: Defect in abdominal wall holding herniated abdominal wall contents.
Dx: Diagnosed on US after week 12 GA (before week 12 herniation of contents physiologic). Hernia covered by amnion & peritoneum; herniation at site of cord insertion. Classified by whether or not defect contains liver (liver-containing defect never nml regardless of GA). Causes elevated MSAFP.
Associations: 50% association w/ cardiac lesion (fetal echo recommended); Beckwith–Wiedemann syn, OEIS syn, & amniotic band syn. Association w/ aneuploidy in nonliver containing lesions (chromo analysis recommended).
• Gastroschisis: Evisceration of abdominal contents through abdominal wall defect.
Dx: Seen as full thickness abdominal wall defect, generally to right of cord insertion (nml cord insertion is seen on US). Bowel may become thickened & matted w/ increasing GA. No overlying peritoneum.
Associations: No ↑ risk of chromosomal aneuploidy but a/w other GI problems. ↑ risk of recurrence w/i families.
• Echogenic bowel: ↑ echogenicity (brightness) of bowel noted on US.
Etiology: A/w bleeding events, aneuploidy, CF, growth restriction, infxn, & idiopathic. Idiopathic = most common etiology.
Aneuploidy: 3–25% association w/ aneuploidy, primarily trisomy 21. Offer amniocentesis for chromosomes, CF, & CMV testing.
Genitourinary Anomalies
• Renal agenesis: Ureteric bud fails to develop & induce differentiation of kidney.
Etiology: Can be bilateral or unilateral. Bilateral usually due to embryonic issue; unilateral difficult to distinguish agenesis from dysplasia & hypoplasia.
Dx: Bilateral renal agenesis diagnosed w/ nonvisualization of kidneys & bladder w/ oligohydramnios. Unilateral diagnosed by absent or abn kidney location (amniotic fluid nml). Full fetal bladder is good indicator of renal fxn.
Prog: Bilateral renal agenesis incompatible w/ life due to pulm hypoplasia. High rate of IUFD due to cord accidents from oligohydramnios.
Associations: 50% association w/ other anomalies; high rate of single umbilical artery
• VACTERL: Vertebral anomalies, Anal atresia, Cardiac defects, TE fistula, Renal defects, Limb defects
• Müllerian anomalies: Defects in female reproductive tract including separate or absent reproductive systems. See Chap. 8.
• OEIS complex: Omphalocele, Exstrophy of the bladder, Imperf anus, Spinal defects
Etiology: Due to abnormalities of cloaca – blind pouch from which rectum & urogenital sinus develop. Typically sporadic & not a/w aneuploidy.
• Bladder exstrophy: Diagnosed w/ absent bladder filling, low-set umbilicus, lower abdominal mass increasing in size throughout Preg. Independent of OEIS complex, can be other assoc abdominal wall, musculoskeletal, & genital deficits.
Musculoskeletal and Anomalies
• Skeletal dysplasias: Qualitatively or quantitatively abn bones on prenatal US.
Dx: FL or HL <5%ile based on GA.
Etiology: Constitutionally short fetus (isolated abn FL), IUGR (a/w small AC), or skeletal dysplasia. Can be marker of aneuploidy.
W/u: Interval growth in 3–4 w can show normalization of FL or nml interval growth. Comparison to other parameters (AC, BPD, HC) can reveal IUGR. If continued short FL compare to qualitative description of other bones.
• Talipes equinovarus (clubfoot): Excessive plantar flexion w/ foot facing medially.
Etiology: Primarily idiopathic or isolated (familial recurrence); can be due to aneuploidy (trisomy 18), deformation (extrinsic).
GENETIC SCREENING
Maternal Serum Aneuploidy Screening (Obstet Gynecol 2007;109:217)
• Aneuploidy screening should be offered to all pts. Counseling includes what is being screened for, potential results, advantages/disadvantages (including cost), & how the results might impact their decisions about the Preg.
• Reported as “risk” of aneuploidy (w/ regard to trisomy 21 & trisomies 13/18) compared to age-matched reference, not as positive or negative (except for cell-free fetal DNA, see below). Overall: 5% positive screen rate (predetermined).
• Screening parameters: Combination of values used in various screening approaches
NT: Defined anatomic area behind fetal neck measured sonographically as width (mm) btw ∼11–14 w. ↑ in aneuploidy & other conditions. Lower false positive rate if combined w/ serum markers. Useful in multiples when serum markers not accurate (ie, each fetus evaluated).
NB: Used w/ NT for trisomy 21 eval
Serum markers: Preg hormones used in combination to calculate risk (AFP, β-hCG, PAPP-A, inhibin A, UE3)
• 1st trimester screening: NT, PAPP-A, & β-hCG in mat serum at 11–14 w. Comparable detection rates to 2nd trimester screen but higher screen positive rate in women >35 yo compared to 2nd trimester screen. Advantages: Time for CVS as diagnostic test & earlier termination options. Disadvantages: More costly approach. In case of sequential strategy, pts must wait for results until 2nd trimester.
• 2nd trimester screening: AFP, hCG, unconjugated estriol (UE3), and inhibin A in screen at 15–18 w & some labs up to 24+ weeks. Detection 69% for triple screen, 81% for quadruple screen (using inhibin A). Advantages: Does not rely on NT (operator dependent test). Serum markers may suggest other problems (eg, ↑ AFP for NTD). Disadvantages: Only screening → amniocentesis for dx. Given later GA, if anomaly found, options may be more limited.
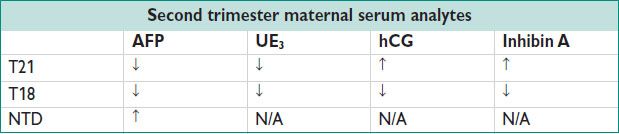
• Combined approaches: Uses both 1st & 2nd trimester screening protocols. When 1st & 2nd trimester protocols used independently, false positive rate ↑.
Integrated screening: Integrates 1st & 2nd trimesters → results given in 2nd trimester
94–96% detection rate w/ full integrated (NT, PAPP-A, quad screen)
Sequential screening: 1st & 2nd trimester screens performed w/ results reported after 1st & then altered after 2nd trimester. Benefits: Allows CVS for those at highest risk & ↓ anxiety of waiting. 95% detection rate by 2nd trimester.
Cell-free Fetal DNA
• Definition: Free fetal DNA in mat circulation likely from syncytiotrophoblast cells, extracted from mat serum, & proportion of target genetic material measured by sequencing. Imbalance of genetic material sugg extra or missing chromo.
• Commercial testing for screening for trisomy 21 & trisomy 18 available. Single bld test w/ >99% sens & spec for T21 & T18. Rapidly evolving technology.
• Applications: Aneuploidy, sex determination (presence of Y chromo), Rh typing. Performed after 10 w.
Screening for Hemoglobinopathies (Obstet Gynecol 2007;109:229)
• Offered to individuals of African, Southeast Asian, & Mediterranean ancestry. If a woman is aware of her status, screening does not need to be repeated. Many US-born women were screened at birth. See Chap. 16.
• Sickle cell: Screen w/ CBC & HbEP in African descent. HbEP allows for detection of HbS & other variants. If positive, partner should undergo carrier screening. Dx: If both partners positive for HbS, refer for genetic counseling to discuss CVS or amniocentesis for diagnostic genetic testing of fetus.
• Thal: Screen w/ CBC & MCV in Southeast Asian & Mediterranean descent
Beta-thalassemia: In pts w/ anemia, MCV < 80, & nml iron status (nml ferritin) HbEP should be performed for screening for thal. HbEP shows elevated HbA & HbF for beta-thalassemia. If positive, partner requires screening.
Alpha-thalassemia: HbEP unable to detect alpha-thalassemia, if of Southeast Asian ancestry w/ microcytic anemia, nml iron studies, & nml HbEP offer DNA testing for abn alpha-globin gene. If positive, partner requires screening.
Dx: If both parents are carriers & have described genetic mutations → offer CVS or amniocentesis for fetal genetic testing
Other Inherited Diseases (Obstet Gynecol 2010;116:1008)
• CF: Autosomal recessive condition due to >1700 of mutations in CFTR gene. Routine testing for common mutations offered to all pts (regardless of ethnicity) after appropriate education regarding the implications of testing & results. Detection rate of test related to prevalence in pop. Pts w/ personal Hx or FHx of CF or related conditions should undergo genetic counseling to determine if expanded mut screens are warranted. If pt positive, partner should be screened & consider amniocentesis/CVS.
• Fragile X: Most common inherited form of MR. Due to ↑ triplet repeats on FMR1 gene. Offer carrier testing in FHx of fragile X-related disorders, unexplained MR, autism, or premature ovarian failure. Variable penetrance based on number of triplet repeats. Only test for FMR1 triplet rpt is diagnostic test using CVS or amniocentesis for known carriers.
• Tay–Sachs: Ashkenazi Jewish, French Canadian, or Cajun descent
• Familial dysautonomia or Canavan dz: Ashkenazi Jewish descent
• Offer other screening tests (musc dystrophy, Huntington’s) based on FHx
AMNIOCENTESIS AND CHORIONIC VILLUS SAMPLING (CVS)
Invasive Prenatal Diagnostic Testing
• Definitive diagnoses for specific conditions. Discuss the difference btw screening & diagnostic tests, risks & benefits, alternate screening tests, & interpretation of results.
Amniocentesis (Obstet Gynecol 2007;110:1459)
• Definition: Removal of AF using transabdominal approach. Procedure performed using spinal needle typically w/ US guidance. For both diagnostic & therapeutic indications. Genetic amniocentesis typically btw 15 & 20 w.
• Diagnostic amniocentesis: Usually for prenatal genetic testing, but several applications.
Genetics: Allows for culture of fetal cells & dx of aneuploidy via karyotype FISH or CGH
Infxn: AF can be used for cell count, gluc, & culture for suspected chorio or can be used to perform diagnostic tests for infxn such as CMV
Hemoglobin: Fetal hemoglobin can be obtained for eval of fetal anemia, fetal bld type, or eval of hemoglobinopathies
Other indications: Can be used to test fetal lung maturity or for NTDs.
• Therapeutic amniocentesis: Amnioreduction (removal of AF) can be therapeutic for pts w/ twin-to-twin xfusion syn & preterm CTX from polyhydramnios.
• Risks: Higher w/ early amniocentesis (11–13 w; not recommended). 1 in 300–500 Preg loss, lower at experienced centers. 1–2% vaginal spotting or LOF; <1:1000 for chorio. AF cells can fail to culture leading to nondiagnosis after amniocentesis. Small risk of transmission of HCV or HBV but data limited. Small risk of transmission of HIV if pt on antiretroviral therapy/undetectable viral load. Rh-negative women should get anti-D RhIg prior to procedure to prevent sensitization
Chorionic Villus Sampling (CVS)
• Definition: Removal of chorionic villi via TA or TC catheter w/ needle under sono guidance. Typically used for dx using karyotype analysis, FISH, or genetic testing for specific alleles. Performed btw 9 & 16 w gest.
• Risks: Complication rate of TA-CVS lower than rates of TC-CVS. Fetal loss (0.7–1.3%) higher than amniocentesis but background rate of fetal loss at earlier GA is higher. Rates of loss at similar GAs are the same btw amniocentesis & CVS. Up to 30% vaginal spotting w/ TC-CVS, less after TA-CVS. Limb reduction or oromandibular defects after 9 w, risk = 6 in 10000 (similar to risk in general pop). Rh-negative women should get anti-D RhIg prior to procedure to prevent sensitization. Nondiagnostic procedure due to operator failure or cell culture failure; higher than for amniocentesis. Higher rate of chromosomal mosaicism (presence of more than one cell line) in CVS compared to amniocentesis (1% vs. 0.25%); if mosaicism, amniocentesis may be indicated. Infxn or leakage of amniotic fluid <0.5%.
• Counseling: Offer to pts interested in 1st trimester diagnostic testing. Advantage of CVS is early GA at dx = more options.
< div class='tao-gold-member'>



