30 Cardiovascular Disorders
 Anatomy and Physiology
Anatomy and Physiology
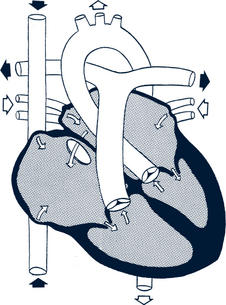
Fetal Circulation
Knowledge of the fetal circulation is essential for understanding the circulatory changes that occur in the newborn at delivery (Fig. 30-1). Fetal circulation has four unique features that differ from postnatal circulation:
• Oxygenation of the blood occurs in the placenta, not the lungs.
• Fetal pulmonary vascular resistance is high, and systemic vascular resistance is low (high pressure on the right side of the heart, low pressure on the left side).
• The foramen ovale, the opening in the septum between the two atria, permits a portion of the blood to flow from the right atrium directly to the left atrium.
• A PDA provides a connection between the pulmonary artery and the aorta that allows blood to flow from the pulmonary artery to the aorta and bypass the fetal lungs.
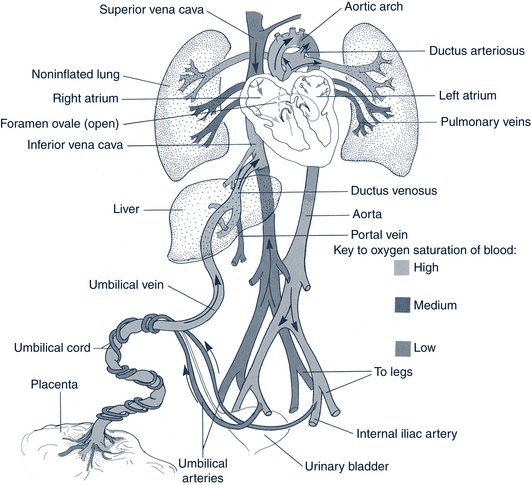
FIGURE 30-1 Fetal circulation.
(From Gorrie TM, McKinney ES, Murray SS: Foundations of maternal-newborn nursing, Philadelphia, 1994, Saunders.)
Neonatal Circulation
A number of complex events occur at birth that rapidly shift the fetal circulation toward a neonatal circulation pattern. Clamping the umbilical cord, with subsequent removal of the placenta as the oxygenating organ, causes an immediate circulatory change in which the lungs become the new source of oxygenation. This change causes an increase in systemic vascular resistance (systemic blood pressure [BP]). With the first breath, mechanical inflation of the lungs and an increase in oxygen saturation bring about a dramatic fall in pulmonary vascular resistance and, consequently, increased pulmonary blood flow. This begins constricting the ductus arteriosus. As the pressures within the heart become relatively higher on the left side and lower on the right, the foramen ovale closes. Functional closure of the ductus arteriosus and foramen ovale usually occurs within the first hours to days of life, and a serial circuit forms out of the once-parallel pulmonary and systemic circulation.
Conduction System
Myocardial contraction is stimulated by electrical depolarization along the conduction tract within the heart. Depolarization begins at the sinoatrial node, which is high in the wall of the atrium. This node acts as the pacemaker of the heart by regularly beginning the depolarizing impulses of each heartbeat. The wave of depolarization travels from the sinoatrial node throughout the atria and produces contraction of the atrial muscle. The impulses reach the atrioventricular (AV) node, which is located in the lower portion of the right atrium at the junction of the atrium and ventricle. From the AV node, the depolarization wave passes through the bundle of His, the fibers extending from the AV node along the intraventricular septum. Depolarization spreads through the left and right branches of the bundle of His and through the Purkinje fibers extending into the ventricular muscle. Impulses then spread throughout the ventricles and cause contraction. The electrocardiogram (ECG) demonstrates this pattern of changing electrical impulses.
 Pathophysiology
Pathophysiology
Table 30-1 lists the most common known chromosomal abnormalities associated with heart disease. Information about ordering genetic tests and resources for families is available from the National Institutes of Health (NIH) website (http://www.nih.gov/) and in-depth information about specific genetic defects or syndromes is available from Online Mendelian Inheritance in Man (OMIM) website (http://www.ncbi.nlm.nih.gov/omim). Genetic testing should also be done in children who have, in addition to CHD, other congenital anomalies, dysmorphic features, neurocognitive deficits, growth retardation, mothers with multiple miscarriages, or siblings with congenital defects (Goldmuntz and Lin, 2008).
TABLE 30-1 Congenital Malformation Syndromes Associated With Selected Congenital Heart Disease
| Disorders | Resultant Heart Defect(s)/Occurrence |
|---|---|
| Syndromes With Chromosomal/Gene Disorders | |
| Trisomy 21 (Down syndrome) | Atrioventricular septal defect, VSD, ASD, PDA, TOF (50%) |
| Trisomy 18 (Edwards syndrome) | VSD, ASD, PDA, COA, bicuspid aortic or pulmonary valve (99%) |
| Trisomy 13 (Patau syndrome) | VSD, PDA, dextrocardia (90%) |
| Turner syndrome (XO) | Bicuspid aortic valve, COA (35%), pulmonic stenosis |
| Marfan syndrome (FBN1 gene) | Mitral valve prolapse, aortic root dilation |
| 22q11.2 deletion syndrome | Interrupted aortic arch, truncus arteriosus, TOF, perimembranous VSD, aortic arch anomalies |
| Klinefelter variant (XXXXY) | PDA, ASD (15%) |
| Williams syndrome | PS, supravalvular AS |
| Noonan syndrome (PTPN11) | Valvular pulmonic stenosis, hypertrophic cardiomyopathy |
| CHARGE (gene CHD7) | Truncus arteriosus, interrupted aortic arch type B |
| Jacobsen (11q23 deletion) | Hypoplastic left heart syndrome, COA |
| Long QT syndrome | Palpitations, syncope, sudden death |
| Holt-Oram syndrome (TBX5) | ASD, VSD |
| Cri du chat syndrome (5p) | VSD, PDA, ASD (25%) |
| Neurofibromatosis | PS, COA |
| Nonhereditary Syndromes | |
| Fetal alcohol syndrome | VSD, PDA, ASD, TOF (25%-30%) |
| Fetal hydantoin syndrome | PS, AS, COA, PDA, VSD, ASD (<5%) |
| Fetal trimethadione syndrome | TGA, VSD, TOF (15%-30%) |
| Infant of diabetic mother | TGA, VSD, COA (3%-5%); cardiomyopathy (10%-20%) |
| Pierre Robin syndrome | VSD, PDA (29%); ASD, COA, TOF (less commonly) |
| VATERL association | VSD, other defects (>50%) |
| Congenital diaphragmatic hernia | VSD, TOF (25%) |
| Cornelia de Lange (de Lange) syndrome | VSD (30%) |
| Other System Malformations | |
| Hydrocephalus | VSD, ECD, TOF (6%) |
| Dandy-Walker syndrome | VSD (3%) |
| Tracheoesophageal fistula and/or esophageal atresia | VSD, ASD, TOF (21%) |
| Imperforate anus | TOF, VSD (12%) |
AS, Aortic stenosis; ASD, atrial septal defect; COA, coarctation of the aorta; ECD, endocardial cushion defect; PDA, patent ductus arteriosus; PS, pulmonary stenosis; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; VATERL, vertebral anomalies, anal atresia, cardiovascular anomalies, tracheoesophageal fistula, esophageal atresia, renal and/or radial anomalies, limb defects; VSD, ventricular septal defect.
Data from Goldmuntz E: The genetic contribution to congenital heart disease, Pediatr Clin North Am 51:1721-1737, 2004; Lin A, Belmont J, Malik S: Heart. In Stevenson R, Hall J, editors: Human malformations and related anomalies, ed 2, Oxford, 2006, Oxford University Press; Park MK, Troxler RG: Pediatric cardiology for practitioners, ed 4, St Louis, 2002, Mosby.
Two percent to 4% of CHD is caused by well-documented teratogens and maternal conditions or environmental influences. Teratogens known to affect the heart include maternal use of thalidomide, cocaine, lithium, fluconazole, phenothiazine, alcohol, anticonvulsants, and retinoic acid. Maternal exposure to pesticides and solvents has also been associated with CHD. Infection, especially cytomegalovirus, mumps, or rubella, contracted in the first 8 weeks of gestation can cause CHD. Infants born to mothers with insulin-dependent diabetes, systemic lupus erythematosus (SLE), and phenylketonuria also have increased risk for various cardiac defects (Goldmuntz and Lin, 2008) (Box 30-1 and Table 30-1).
BOX 30-1 Risk Factors Suggestive of Congenital Heart Disease
Perinatal Risk Factors
Maternal infections and exposures (CMV, rubella, other viral syndromes)
Maternal use of tobacco, alcohol, street drugs, retinoic acid, hydantoins, lithium, valproates
Maternal chronic disease (CHD, lupus, insulin-dependent diabetes, phenylketonuria)
Maternal age at child’s birth (increase in chromosomal abnormalities after 40 years old)
Maternal pregnancy history (excessive weight gain, gestational diabetes)
Neonatal Risk Factors
Fetal or newborn distress (aspiration, hypoxia, cyanosis)
Prematurity (increased incidence of CHD in premature infants)
Presence of associated anomalies (genetic or chromosomal abnormalities or syndromes)
Birthweight (term infants, <2500 g; SGA, <2 standard deviations from the mean for gestational age)
Newborn Risk Factors
Murmur at birth or early infancy
Hypertension (at birth or beyond)
Feeding difficulty (SOB, easily fatigued, diaphoresis, poor intake)
Cyanosis (increase with crying, feeding, exertion)
Toddler, School-Age, and Teenage Risk Factors
Frequent respiratory tract infections (pneumonia, URIs that last longer than normal)
Hypertension (documented on a minimum of three separate visits)
SOB with exertion (beyond normal peers)
Syncope or dizziness (especially associated with noted heart rate change)
Tachycardia or bradycardia (fluttering in chest, racing heart)
 Assessment of the Cardiovascular System
Assessment of the Cardiovascular System
History
Cardiac evaluation includes review of the family, maternal, fetal, neonatal, and infant medical history, in addition to growth and development (see Box 30-1 for risk factors).
Physical Examination
Physical assessment in a child with suspected CHD should be adapted to the age of the child (Box 30-2). Be flexible, yet thorough, in any evaluation and include all aspects of the physical examination in an order that best suits the comfort and needs of the infant, child, or adolescent.
BOX 30-2 Developmental Approach to Cardiac Assessment
Infants
Perform uncomfortable aspects of the examination after auscultation to ensure a quiet listen.
Observe color, respiratory effort, and general effort level while the baby is quiet.
Toddlers
Allow the child to handle a stethoscope while the history is being taken.
Have a toy or distraction item available during the examination.
Consider “listening” to the parent’s heart first to improve comfort with the examination.
School Age
Clearly explain the plan and expectations before the examination.
Answer the child’s questions honestly.
Talk about topics of interest (school, sports) during the examination.
School-age children may be modest and prefer to keep a gown on during most of the examination.
Adolescents
Questions should be directed at the adolescent and parent.
Communicate in a manner that conveys honesty, professionalism, and interest in their concerns.
Ensure privacy related to both the physical examination and information sharing.
Provide a choice of having a parent present for any or all aspects of the history and examination.
Vital Signs
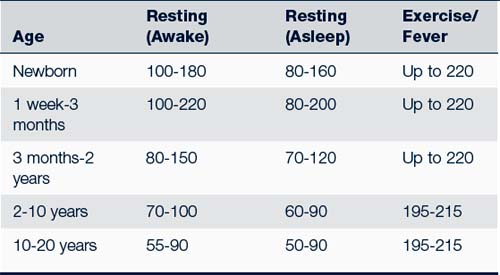
• Heart rate (Table 30-2). Heart rates should always be obtained by auscultation of the heart in children younger than 10 years old. Assessment should include rate and rhythm variations. An increased heart rate can be caused by excitement, anxiety, hyperthyroidism, heart disease, anemia, or fever. Assess the rhythm for regularity.
• Pulses. Pulses should be checked in the upper and lower extremities and evaluated for character (strength) and variation between the different sites. A bounding pulse may indicate a PDA or aortic insufficiency. Weak or “thready” pulses may indicate CHF or an obstructive lesion, such as severe AS. Good brachial pulses in conjunction with weak or absent femoral pulses may indicate coarctation of the aorta (COA).
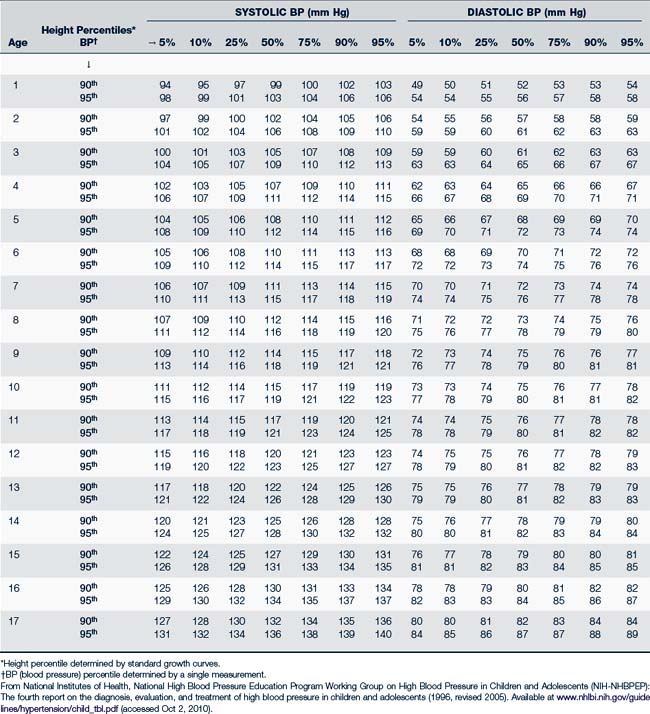
• BP. The National Institutes of Health National High Blood Pressure Education Program (NIH-NHBPEP) (2004) on BP control in children and adolescents recommends measuring BP annually beginning at 3 years of age. Providers should auscultate BP on children 3 years or older. In selected cases, in which the index of suspicion of heart disease is high, providers should check BPs in younger children. It is important to always use a BP cuff that is appropriate for the child’s size. For arm pressure, the width of the cuff should be two thirds the length of the upper arm measured from the axilla to the antecubital space. A cuff that is too narrow or does not fit around a chubby arm may cause an erroneously high reading. Cuff sizes of 3, 5, 7, 12, and 18 cm should be on hand in order to accommodate the array of pediatric patient sizes. Initial evaluation should compare the pressure in all four extremities. Pressure in all extremities should be equal, with pressure in the legs being slightly higher (10 to 20 mm Hg) in a child who walks. Lower extremity pressure is measured with the stethoscope placed over the popliteal artery. The NIH periodically publishes norms for BP by gender, age, and height; they are found in Tables 30-3 and 30-4 (NIH-NHBPEP, 2004).
• The pulse pressure (difference between systolic and diastolic pressure) is normally 20 to 50 mm Hg throughout childhood. A wide pulse pressure caused by an abnormally low diastolic pressure may be an indication of PDA, aortic regurgitation, or other cardiac pathologic conditions.
• Respiratory rate. Evaluation of the respiratory system includes the respiratory rate, assessment of effort, and breath sounds in all five lobes of the lungs. It is important to evaluate the respiratory rate in a quiet infant or child. A respiratory rate greater than 40 in a young child or 60 in a newborn who is quiet, resting, and afebrile warrants further evaluation. An infant with CHD may be happily tachypneic and not show significant signs of grunting, intercostal retractions, nasal flaring, or tracheal tug (up and down movement of the trachea with each inspiration).
• Oxygen saturation. Oxygen saturation is considered to be an essential vital sign in many settings. It is important to obtain oxygen saturations in new babies or new patients because cyanosis is often subtle and not always readily perceptible to all examiners. Pulse oximetry alone may detect cyanotic heart disease in asymptomatic newborns, preventing possible mortality and morbidity from delayed diagnosis (Mahle et al, 2009).
TABLE 30-3 Blood Pressure Levels in the 90th and 95th Percentiles for Girls 1 to 17 Years Old by Percentiles of Height
General Appearance
• During this observation period note the presence of unusual facial characteristics (e.g., malformed ears, wide-spaced eyes, noticeable anomalies) or extracardiac anomalies (e.g., cleft lip or palate, polydactyly, microcephaly) that may be associated with a syndrome or chromosomal abnormalities. Children may have obvious stigmata, such as those seen with Down syndrome, Marfan syndrome (unusually tall with an arm span wider than the head-to-toe height), Turner syndrome (webbed neck, prominent ears), or fetal alcohol syndrome (microcephaly and pinched facies), all of which are associated with CHD.
• Overall skin color should be assessed for signs of mottling or central cyanosis while the infant is at rest. Cyanosis caused by heart disease is recognized as a pale blue or ruddy red color of the mucous membranes (lips, tongue, nailbeds). The tongue is the best indicator because it lacks pigmentation and is abundantly served by the vascular system. Peripheral cyanosis or acrocyanosis, a blueness or pallor noted around the mouth and on the hands or feet, can be a normal variant, especially if it intensifies when the infant is cold. Clubbing of the fingers and toes may be seen in children with long-standing cyanosis.
• Note any wheezing, nasal flaring, retractions, prominent neck veins, or head bobbing with respirations.
• Note also signs of peripheral or periorbital edema. Edema or puffiness around the eyes may be evident in an infant with CHF even in the absence of peripheral edema of the hands or feet. True pitting edema of the feet is an unusual finding in an infant with CHF.
• Measure and plot height and weight on standardized charts, including Down and Turner syndrome charts, at each assessment. Although many children with CHD fall within the normal ranges of height, weight, and development, a large number of infants and children with heart disease experience poor weight gain, less than normal linear growth, and delays in achieving developmental milestones.
Palpation
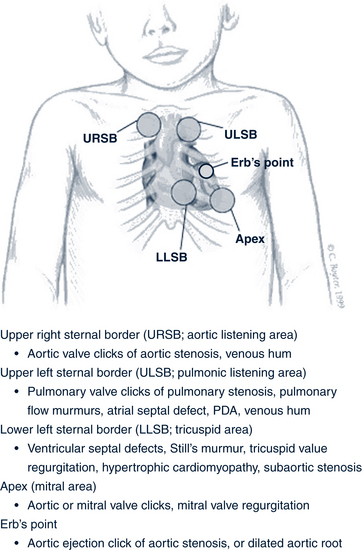
Palpate all five areas of the chest: the aortic, pulmonic, tricuspid, and mitral areas, and Erb’s point (Fig. 30-2). Chest palpation is best accomplished by using the open palm of the hand near the base of the fingers. The hand should be gently moved across the chest to assess abnormal precordial activity, including pulsations, lifts, heaves, or thrills, and to determine the location of the apical impulse. The apical impulse is used to determine the size of the heart and is the most lateral point at which cardiac activity can be palpated. In infants and children, the impulse is normally palpated at the apex of the heart in the fourth intercostal space just to the left of the midclavicular line. At approximately 7 years old, the point shifts to the fifth intercostal space. In the presence of cardiomegaly, the apical impulse is shifted laterally or downward.
• Thrills are a palpable vibration caused by turbulent blood flow through abnormal structures or defects in the heart. The turbulent flow may be due to valvular narrowing or stenosis or defects, such as VSD.
• Assess peripheral pulses (radial, brachial, carotid, dorsalis pedis, and posterior tibial) for amplitude and intensity. In COA, the examiner will note decreased or absent femoral pulses and impulse lag if the radial pulse is palpated simultaneously. A fast pulse rate may indicate arrhythmia or CHF.
• The liver and spleen should be assessed for enlargement. A liver more than 1 cm below the right costal margin in an older infant or child may indicate hepatomegaly and is an important finding. Infants may have a palpable liver edge as a normal finding.
• The back should be examined for scoliosis, a finding associated with enlarged hearts.
Auscultation of Heart Sounds
• The examiner should approach auscultation of the heart in the same manner for every child either beginning at the base or apex of the heart. Ideally, assess heart sounds in a quiet environment when the child is cooperative.
• Four individual heart sounds can be heard: S1, S2, S3, and S4. S1 and S2 represent normal heart sounds, whereas the presence of S3 or S4 may indicate cardiac enlargement or volume overload.
• At each area of examination, the provider should accurately identify the first (S1) and second (S2) heart sounds.
• S1 has the following characteristics:
 It may be differentiated from early systolic clicks by the low frequency of the sound (clicks have a higher frequency). It is best heard with the diaphragm of the stethoscope.
It may be differentiated from early systolic clicks by the low frequency of the sound (clicks have a higher frequency). It is best heard with the diaphragm of the stethoscope.• S2 has the following characteristics:
 S2 is normally split with inspiration because pulmonic valve closure lags behind aortic valve closure. S2 becomes single with expiration.
S2 is normally split with inspiration because pulmonic valve closure lags behind aortic valve closure. S2 becomes single with expiration. Pulmonary hypertension causes early closure of P2 and accentuation of S2, which may sound like a loud, single second heart sound.
Pulmonary hypertension causes early closure of P2 and accentuation of S2, which may sound like a loud, single second heart sound. Wide splitting of S2 without becoming a single sound on expiration may indicate increased pulmonary flow (typical of ASD).
Wide splitting of S2 without becoming a single sound on expiration may indicate increased pulmonary flow (typical of ASD).• S3 and S4 have the following characteristics:
 S3 is associated with rapid ventricular filling; it may be heard in a quiet infant or child with a rapid heart rate.
S3 is associated with rapid ventricular filling; it may be heard in a quiet infant or child with a rapid heart rate. S3 “gallop” is best heard at the apex with the bell of the stethoscope during early diastole. When combined with S1 and S2, it gives an impression of the word “Kentucky.” S3 is easier to appreciate when the child is in the left lateral decubitus position.
S3 “gallop” is best heard at the apex with the bell of the stethoscope during early diastole. When combined with S1 and S2, it gives an impression of the word “Kentucky.” S3 is easier to appreciate when the child is in the left lateral decubitus position. S4 is always pathologic; it represents increased force of atrial contraction and ventricular distention.
S4 is always pathologic; it represents increased force of atrial contraction and ventricular distention.• Clicks: Ejection clicks are heard early in systole, immediately after S1, and may sound like a split first heart sound. Pulmonic ejection clicks are high in frequency, vary with respiration, and disappear with inspiration. An aortic ejection click, heard best at Erb’s point, is constant in intensity with a sound of a “snap” or a “click.” Nonejection clicks are heard best in midsystole, or midway between S1 and S2 in the cardiac cycle at the apex. These clicks are best heard in patients who are leaning forward or standing, may disappear with inspiration, and are due to mitral valve prolapse (Park, 2008). Figure 30-2 describes cardiac conditions associated with each of these clicks.
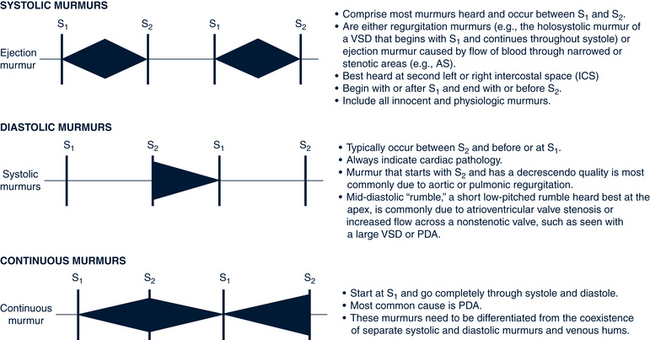
Murmurs
Innocent or Functional Murmurs
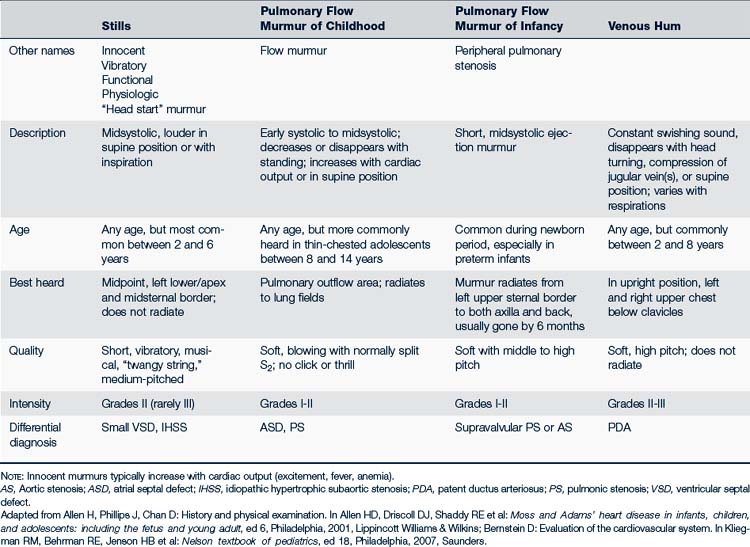
Functional or innocent cardiac murmurs are common in children and can be evident in newborns. Table 30-5 describes common types of innocent murmurs; Box 30-3 describes the characteristics of an innocent murmur. Families and older children with innocent murmurs should be reassured that there is no cardiac pathology. They should be informed that this murmur may come and go and may be louder at times of fever, anxiety, pain, or exercise, and that activities do not need to be limited or any special precautions taken.
BOX 30-3 Auscultatory Findings—The Innocent Versus Pathologic Murmur
The Innocent Murmur
• Usually grade I-II/VI in intensity and localized
• Changes with position (sitting to lying)
• May vary in loudness or presence from visit to visit
• May increase in loudness (intensity) with fever, anemia, exercise, or anxiety
• Musical or vibratory in quality
• Systolic in timing except for venous hum, which is continuous
• Best heard in LLSB or pulmonic area (except for venous hum)
• May disappear with Valsalva maneuver, position, or gentle jugular pressure
Possible Pathologic Murmur: Refer these to a pediatric cardiologist
• A murmur in a patient with a syndrome known to have a high incidence of CHD (e.g., trisomy 21)
• Any systolic murmur that is associated with a thrill
• Continuous murmurs that cannot be suppressed
• Fixed splitting of the second heart sound not associated with bundle branch block
CHD, Congenital heart disease; ECG, electrocardiogram; LLSB, left lower sternal border.
Data from Lucas JF, Saul JP: Innocent murmurs. In Burg FD, Ingelfinger JF, Polin RA et al, editors: Current pediatric therapy, ed 18, Philadelphia, 2006, Saunders.
Criteria for Describing a Heart Murmur
Every murmur is assessed according to the criteria listed in Table 30-6. These are further illustrated and discussed in Figure 30-3. Characteristics of pathologic murmurs needing referral are listed in Box 30-3. The presence of a murmur causes great anxiety for a family awaiting a diagnosis. All murmurs should have a second opinion from a pediatric colleague or pediatric cardiologist if the diagnosis is uncertain or there is a suspicion of heart disease (Allen et al, 2008).
TABLE 30-6 Describing a Heart Murmur
| Grade or intensity: | Grade I: Barely audible; heard faintly after a period of attentive listening Grade II: Soft but easily audible Grade III: Moderately loud, no thrill Grade IV: Loud, present Grade V: Loud, audible with stethoscope barely on the chest Grade VI: Heard without stethoscope (rare) |
| Timing with cardiac cycle | Systolic Diastolic Continuous |
| Location on chest where murmur is loudest | Aortic or pulmonic listening areas, URSB, ULSB, Erbs point, LLSB, apex |
| Radiations or transmission to other locations | To back To apex To carotids |
| Quality | Musical Harsh blowing |
| Duration | Point of onset and length of time systole and diastole murmurs last (e.g., “early systole, heard throughout cardiac cycle”) |
| Pitch | Low Middle High |
LLSB, Left lower sternal border; ULSB, upper left sternal border; URSB, upper right sternal border.
Common Diagnostic Studies
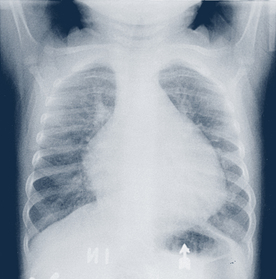
• Chest radiograph. Radiography provides the following information: cardiac size and size of specific chambers and great vessels, cardiac contour, status of pulmonary blood flow, and status of the lungs and other surrounding tissue (Fig. 30-4).
• ECG. ECG monitors the electrical activity of the heart from different locations and in different planes of the body. The ECG gives information about forces of ventricular contraction, hypertrophy, chamber dilation, and rhythm.
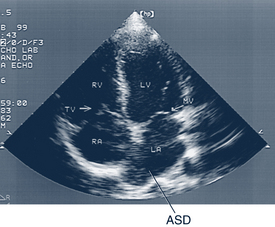
• Echocardiogram. Echocardiography uses reflected sound waves to identify intracardiac structures and their motion. The types of recordings include two-dimensional, M-mode, contrast, Doppler, and tissue Doppler studies (Fig. 30-5). Fetal echocardiography can diagnose CHD as early as 16 to 18 weeks’ gestation (high-frequency transvaginal echocardiography as early as 10 weeks’ gestation), as well as dysrhythmias and hemodynamic changes (Kleinman et al, 2008).
• Complete blood count (CBC). CBC rules out severe anemia or polycythemia as a cause of a murmur.
Other diagnostic tests may include the following:
• Cardiac catheterization. An opaque catheter is introduced into the heart chambers via a large peripheral vessel. The cardiologist measures pressures and saturation in chambers and vessels and uses dye to outline anatomy. This provides information about cardiac output, vascular resistance, and the response of the heart to exercise and medications.
• Hyperoxia test. Supplementation of 100% oxygen results in “pinking” and increased arterial oxygen saturation when the disease is primarily pulmonary; minimal or no color improvement indicates that the disease is cardiac. More commonly, simple pulse oximetry saturations are used to evaluate for cyanosis.
• Magnetic resonance imaging (MRI). This technique uses a strong magnetic field to cause movement of nuclei to yield an image of the heart structures and information about chamber volumes and function.
• Exercise testing. A graded treadmill or bicycle ergometer is used to determine cardiac output (myocardial blood flow and rhythm) response to exercise for endurance and capacity measurement.
 Management Strategies
Management Strategies
Primary Health Care for Children with Cardiovascular Diseases
The goals of primary health care for a child with cardiovascular disease include the following:
• Adequate nutritional intake and optimal growth. Depending on the child’s condition, the family may need help in modifying the diet to provide maximum calories or limit various types of foods. The young infant with CHF may need 24, 27, or 30 kilocalories per ounce of formula or fortified breast milk. The child may also need a nasogastric or gastric tube to obtain adequate calories because he or she may be unable to suck adequately. Children with cyanotic conditions may initially have adequate weight gain. The provider should refer to a nutritionist if available for assistance with complex diets (see Chapter 10 for more detailed information).
• Optimal psychosocial development and functioning. Discuss with the family the need to treat the child as normally as possible. Direct parents to support groups that provide informational and emotional support for families. Provide support to siblings of the affected child as well. Poor sibling bonding and unexpressed fears and anger in young siblings toward an infant with a severe or chronic disease can affect their relationships and family dynamics for many years. A retrospective review of studies that pertained to the long-term psychological adjustment of children and adolescents following open heart surgery for CHD concluded that these survivors are at risk for psychological maladjustment and impaired health-related quality of life (Latal et al, 2009).
• Optimal preventive and primary health care. Live virus vaccines should be delayed until 6 to 7 months after cardiopulmonary bypass (and exposure to red blood cells and plasma) or immune globulin exposure (AAP, 2009). This most often affects 1-year-old infants who are due for varicella and measles, mumps, and rubella vaccines. Other vaccines can be given on a regular schedule. The AAP recommends provision of respiratory syncytial virus (RSV) prophylaxis for infants less than 2 years old who have cyanotic or complicated CHD. (Refer to the current AAP Red Book or Centers for Disease Control and Prevention [CDC] website for immunization schedule.)
• Prevention of avoidable complications. Prevention of respiratory infections through good handwashing and avoiding contact (if possible) with others with upper respiratory infection (URI) symptoms, should be emphasized. Vaccination against seasonal influenza is prudent.
• Prevention of infective endocarditis (IE). Although uncommon in children, IE (also called subacute bacterial endocarditis [SBE]) is associated with a high morbidity and mortality rate (discussed later in this chapter) and warrants primary prevention whenever indicated. Standards for prophylaxis against SBE are available in Box 30-4, and Tables 30-7 and 30-8). A high index of suspicion for IE should be maintained if any unusual clinical findings (e.g., petechiae, fever) are present after any procedure. Children with CHD appear to have more severe gingival inflammatory conditions, with a concomitant increase in Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species (HACEK) and other microbes known to cause endocarditis compared with other children (Steelman et al, 2003). The reason for this is not clear. Good dental hygiene is extremely important for these patients.
• Optimal fitness. Reassure the parents that the child generally “self-limits” activity according to ability. The cardiology provider should be consulted regarding exercise limitations before entrance into sports or any activities that require strenuous physical exertion. Parameters for sports participation for children with various forms of cardiac diseases or conditions can be found in Chapter 13, Table 13-5.
• Optimal neurodevelopmental adaptation to school and life tasks. Several studies have shown relatively high incidences (up to 50%) of neurodevelopmental impairments in school-age children who had open heart surgery in infancy (Majnemer et al, 2008). Although mean intelligence scores are generally within the average range, many of these children have difficulties with visuospatial tasks, fine motor functions, higher order language skills, memory and attention. They may be impaired in their ability to coordinate lower-order skills to perform higher-order tasks. In one study, 25% of children who had neonatal heart surgery had developmental disabilities. A few studies show that there is brain injury and immature brain development in infants with CHD prior to heart surgery, presumably due to disordered fetal circulation (Miller el al, 2007).
BOX 30-4 Cardiac Conditions Associated With the Highest Risk of Endocarditis: Prophylaxis Recommended
Prophylaxis is recommended for individuals who have had:
• Procedures that involved the application of prosthetic valves
• Procedures that involved the use of prosthetic material to repair cardiac valves
• A history of infective endocarditis
• Unrepaired cyanotic congenital heart disease, including palliative shunts and conduits
• Completely repaired congenital heart defect with prosthetic material or device(s) whether via surgery or catheterization for the first 6 months after the procedure due to the endothelialization of prosthetic material within that period
• Repaired congenital heart disease with residual defects (e.g., residual ventricular septal defect) at the site of or adjacent to an area of a prosthetic patch or prosthetic device
• A cardiac transplant and then develop cardiac valvulopathy
Data from Nishimura RA, Carabello BA, Faxon DP et al: ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons, Circulation 118:887-896, 2008.
TABLE 30-7 Procedures for Which Endocarditis Prophylaxis Is or Is Not Recommended in High-Risk Individuals
| Prophylaxis Recommended | Prophylaxis Not Recommended | |
|---|---|---|
| Dental | Dental extractions Periodontal procedures Dental implant placement and reimplantation of avulsed teeth Root canal instrumentation Initial placement of orthodontic braces but not brackets Teeth or implant cleaning where bleeding is expected Postoperative suture removal | Restorative dentistry Routine anesthetic injections through noninfected tissue Intracanal endodontic treatment; postplacement and buildup Rubber dam placement Placement of removable prosthodontic or orthodontic appliances Bleeding from trauma of lips or oral mucosa Taking oral impressions Fluoride treatments Taking oral radiographs Orthodontic appliance adjustment or placement of brackets Shedding of primary teeth |
| Respiratory tract | Tonsillectomy or adenoidectomy Surgery involving respiratory mucosa | Endotracheal intubation Flexible bronchoscopy without biopsy Pressure tympanostomy tube insertion/removal Rigid bronchoscopy |
| Skin | Procedures involving infected skin or skin structures | Uncomplicated skin biopsy Tattooing (but this is highly discouraged in high-risk individuals) |
| Musculoskeletal tissue | Procedures involving infected musculoskeletal tissue | |
| Genitourinary tract | Cystoscopy or urinary tract manipulation in patients with enterococcal infection | Vaginal delivery Cesarean section Urethral catheterization without infection Therapeutic abortion Circumcision Hysterectomy |
| Gastrointestinal tract | GI tract procedures including esophagogastroduodenoscopy or colonoscopy | |
| Other procedures | Cardiac catheterization, including device placement and pacemakers |
Data from Nishimura RA, Carabello BA, Faxon DP et al: ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons, Circulation 118:887-896, 2008.
TABLE 30-8 Prophylactic Regimens for Dental Procedures*
| Route | Agent | Regimen† |
|---|---|---|
| Able to take oral medication | Amoxicillin | Adults: 2 g PO; children: 50 mg/kg PO (max 2 g) |
| Unable to take oral medication | Ampicillin or‡ | Adults: 2 g IM or IV; children: 50 mg/kg IM or IV (max 2 g) |
| Cefazolin or ceftriaxone | Adults: 1 g IM or IV; children: 50 mg/kg IM or IV (max 1 g) | |
| If penicillin allergic, oral | Clindamycin or‡ | Adults: 600 mg PO; children: 20 mg/kg PO (max 600 mg) |
| Cephalexin or cefadroxil or | Adults: 2 g PO; children: 50 mg/kg PO (max 2 g) | |
| Azithromycin or clarithromycin | Adults: 500 mg PO; children: 15 mg/kg PO (max 500 mg) | |
| Penicillin allergic and unable to take oral medication | Clindamycin or‡ | Adults: 600 mg IM or IV; children: 20 mg/kg IM or IV (max 600 mg) |
| Cefazolin or ceftriaxone | Adults: 1 g IM or IV; children: 50 mg/kg IM or IV (max 1 g) |
IM, Intramuscular; IV, intravenous; max, maximum, PO, orally.
* Antibiotic regimens are procedure specific; refer to the American Heart Association reference for nondental prophylaxis recommendations.
† Take 30-60 minutes prior to the procedure.
‡ Or other first- or second-generation oral cephalosporin in equivalent adult or pediatric dosage. Cephalosporins should not be used if there is a history of anaphylaxis, angioedema, or urticaria with penicillins.
Data from Nishimura RA, Carabello BA, Faxon DP et al: ACC 2008 guideline update on valvular heart disease: focused update on infective endocarditis. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons, Circulation 118:887-896, 2008.
 Congenital Heart Diseases
Congenital Heart Diseases
Congestive Heart Failure
CHF refers to a progressive clinical and pathophysiologic syndrome found in many children with heart problems. The symptoms vary with age of the child and the root cardiac problem. Box 30-5 provides information on age specific signs and symptoms of CHF. Besides functional changes, CHF is marked by changes in neurohormonal and molecular changes within the heart (Hsu and Pearson, 2009a).
CHF in children can be caused by congenital malformations leading to volume overload (such as a large VSD) or pressure overload (such as AS) or more complex heart disease. CHF can also occur in children with structurally normal hearts due to cardiomyopathy or secondary to dysrhythmias, ischemia, toxins, or infections (Table 30-9). These conditions are discussed in more detail later in this chapter. One set of authors estimate CHF affects 12,000 to 35,000 children each year (Hsu and Pearson, 2009a).
TABLE 30-9 Conditions That Can Lead to Congestive Heart Failure in Children
| Age | Condition |
|---|---|
| Premature infant | Patent ductus arteriosus |
| Birth to 1 week | Hypoplastic left heart syndrome |
| Coarctation of the aorta | |
| Critical aortic stenosis | |
| Interrupted aortic arch | |
| Arteriovenous malformations | |
| Tachycardia | |
| Cardiomyopathy | |
| 1 week to 3 months | Ventricular septal defect |
| Truncus arteriosus | |
| Atrioventricular canal (endocardial cushion defect) | |
| Total anomalous pulmonary venous return | |
| Coarctation | |
| Tachycardia | |
| Patent ductus arteriosus | |
| Aortic stenosis | |
| Tricuspid atresia | |
| Older than 1 year | Bacterial endocarditis |
| Rheumatic fever | |
| Myocarditis |
A greater understanding of the underlying neurohormonal mechanisms of CHF has been detailed and aided by therapies in the adult population. Depending on the underlying pathophysiology, the same patterns of elevated neurohormonal and inflammatory mediators (aldosterone, norepinephrine, natriuretic peptides, tumor necrosis factor, and renin) occur in children. In adult populations large-scale studies have shown the value of blocking some of the chronic neurohormonal changes to CHF with agents such as aldosterone inhibitors, angiotensin inhibitors, and sympathetic inhibitors (beta-blockers). To date, however, no large-scale pediatric study has been able to show positive effects with this change in CHF therapeutic focus. However, based on adult evidence and some small pediatric studies, the International Society of Heart and Lung Transplantation recommended angiotensin-converting enzyme (ACE) inhibitors for moderate to severe left ventricular dysfunction (Hsu and Pearson, 2009b). The traditional armamentarium of heart failure therapies (i.e., diuretics, inotropes, and afterload reducers) is likely to be added in the future after further elucidation of the neurohormonal responses in children with the different conditions listed in Table 30-9. The role of monitoring B natriuretic peptides (amino acid polypeptides secreted by the ventricles in response to stretching) in managing CHF is also evolving.
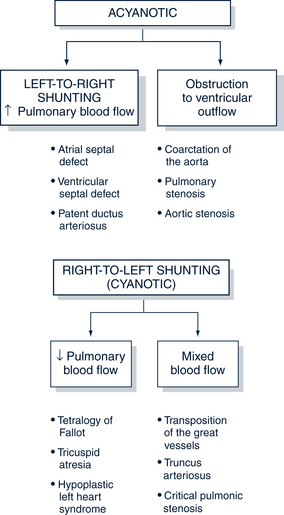
Left-to-Right Shunting Congenital Heart Disease (Acyanotic)
Pulmonary overflow lesions have a communication between the two sides of the heart through which extra blood shunts from the high-pressure, oxygenated left side of the heart to the low-pressure, deoxygenated right side of the heart. The result is an increase in pulmonary blood flow (Fig. 30-6). These lesions are acyanotic in nature. Table 30-10 illustrates the distinguishing features of left-to-right versus right-to-left shunting disorders.
Atrial Septal Defect
Description and Epidemiology
An ASD is a defect or hole in the atrial septum. Of the four types of ASD, the most common involves the midseptum in the area of the foramen ovale and is called an ostium secundum–type defect (Fig. 30-7). Defects of the sinus venosus type are high in the atrial septum, near the entry of the SVC, and are frequently associated with anomalous pulmonary venous return. A primum ASD is in the lower portion of the septum and is most often seen in children with Down syndrome. The rarest form of ASD is an unroofed coronary sinus (see Table 30-10 for incidence figures). Usually ASDs occur spontaneously; however, there are a few identified genetic mutations which cause familial ASDs (Porter and Edwards, 2008).