INTRODUCTION
As our knowledge of the genetic, physiologic, environmental, and lifestyle factors associated with the carcinogenic process grows, the prevention of cancer is increasingly becoming a reality and being incorporated into oncology practice. Options for the primary prevention of cancer are expanding and include avoidance of carcinogens (e.g., smoking cessation, sun avoidance, removal of asbestos), diet modification, exercise, and weight loss, use of cancer vaccines, prophylactic surgery, and chemoprevention. It is estimated that avoidance of tobacco, sun exposure, and elimination of obesity could have a major public health impact on cancer incidence, as well as other chronic conditions. Options for secondary prevention through screening for occult disease when treatment may be more effective are also becoming more sophisticated. Great strides have been made in the prevention of many of the most common cancers.
The most common risk factor for lung cancer, which is the largest cause of death from cancer for both men and women, is tobacco use. Primary prevention strategies, including tax increases on tobacco products, restrictions on smoking in public places, and the physiologic and psychologic treatment of nicotine addiction have all contributed to the reduction in smoking prevalence rates and the decrease in the incidence of lung cancer (1, 2). Recently, low-dose spiral CT scans have been shown to detect early-stage lung cancer and result in a reduction in lung cancer mortality (3, 4).
Nonsteroidal anti-inflammatory drugs reduce the risk of adenomatous polyps and invasive cancer among individuals with very high risks of colon cancer due to hereditary syndromes (5). Their use for primary prevention among individuals at average risk, however, is not recommended due to potential side effects. Perhaps the greatest contribution to the prevention of colorectal cancer is the increasing adoption of screening colonoscopy to identify and remove premalignant polyps (6).
Large randomized trials have established the efficacy of both tamoxifen and raloxifene to prevent estrogen receptor positive breast cancer (7, 8). With the demonstration of the increased risk in breast cancer associated with combined estrogen and progesterone hormone replacement therapy by the Women’s Health Initiative, the use of these products has declined significantly, and the decline is thought to be associated with a subsequent decrease in breast cancer incidence (9, 10). Both prophylactic mastectomy and prophylactic oophorectomy reduce the risk of breast cancer and are considered options for women with hereditary syndromes that convey high rates of breast and ovarian cancer (11). Advances in screening modalities for breast cancer, including the increasing adoption of digital mammography and the use of screening breast MRI in selected high-risk individuals has improved the detection of early-stage occult cancers, and has led to a down staging of disease (12, 13).
A large randomized trial of finasteride, which reduces the androgenic stimulation of the prostate, produced a 25% reduction in prostate cancer incidence (14). Several other agents have been proposed as chemopreventive agents for prostate cancer, but so far none have proven efficacy. The role of prostate cancer screening with digital rectal exam and serum PSA levels among average risk men has recently been challenged (15). The role of screening high-risk men, African American men and those with a family history of this cancer, is the subject of ongoing studies.
The prevention of gynecologic cancer is becoming a reality due to the recognition that the initiation and progression of gynecologic cancers is a multistep process characterized by distinct molecular genetic events that provide opportunities to intervene in the carcinogenic process at several steps and reverse its early stages. The concept of preventing gynecologic cancer is based on an understanding of causally related risk factors, their role in carcinogenesis, and opportunities for their avoidance and/or reversal of their effect. There are 3 distinct models that can be applied to gynecologic cancer prevention: (a) risk avoidance and adoption of protective practices includes the identification of key risk factors and the development of strategies for their avoidance. Included in risk avoidance are the avoidance of exogenous and endogenous exposures (chemical, hormonal, infectious, etc.) and the avoidance of risky health behaviors. The adoption of protective practices, such as vaccination with the human papillomavirus (HPV) vaccine, a healthy diet, and exercise, may forestall early premalignant events; (b) the use of chemopreventive agents, both natural and synthetic, to reverse early, premalignant changes; and (c) surgical prophylaxis to remove either healthy at-risk organs or tissues with premalignant changes.
In addition to establishing valid interventions for cancer prevention, it is important to identify optimal target populations for their application, and to tailor the interventions to the level of risk. Interventions for use in the general population at average risk must be highly effective, safe, inexpensive, and socially acceptable. Population groups with high risks may tolerate interventions that confer more risk and higher costs. All prevention efforts are greatly enhanced by public and professional education about their use and by a health care system that values, promotes, and invests in prevention activities.
The avoidance of environmental, occupational, and lifestyle risk factors through public education and social policies has the potential to prevent a large proportion of human cancer. The epidemiological literature has provided a wealth of information about the risks associated with cancers of the cervix, uterus, and ovary, which allows us to devise risk avoidance and risk reduction strategies. This chapter focuses on the opportunities for primary prevention of these three cancers and directions for the future.
CERVIX CANCER
Risk Factors
There are approximately 13,000 new cases of cervical cancer per year in the United States, and over 4,000 women die of the disease. The disease burden is not distributed equally but is overrepresented in the United States among African American, Hispanic/Latina, and American Indian women (16). The greatest burden of cervical cancer is in the developing countries (Fig. 6.1), which account for 86% of the cases and 88% of deaths (17). Traditionally, the most significant factor associated with the risk of cervical cancer has been number of sexual partners and the early onset of sexual activity. This observation has led to the discovery that the primary cause of cervical cancer, and its precursor, intraepithelial lesions, is persistent infection with HPV, which is sexually transmitted. The HPVs are a large (over 100 types) family of viruses that infect skin and mucosa (Fig. 6.2). Of the approximately 40 HPV types that infect the genital tract, about one-half are associated with anogenital warts and are considered low risk for malignancy, or nononcogenic. The other half may give rise to a range of anogenital cancers, including cervix, vulva, and anus in women, and penis and anus in men, and are referred to as high-risk or oncogenic (18). HPV types 16 and 18 alone account for >70% (Fig. 6.3) of all cervical cancers. Similarly, HPV types 6 and 11 account for >90% of all anogenital warts. In the United States, where the median age of sexual debut is 17 years, close to 20% of girls are sexually active by age 15 years, and close to 60% are sexually active at age 18 years. As a result, the infection rate of HPV among the general population is high, peaking in the second and third decades of life when infection rates range from 27% to 46% (19). The median length of infection is 8 to 12 months, and most individuals have cleared the virus by 2 years. A small proportion, 10% to 13%, however, develop chronic persistent HPV infection, which can lead to genital warts, cervical dysplasia, carcinoma in situ, and invasive cancer (Fig. 6.4). Persistence of HPV infection is likely to be related to modifying factors, including immune status, the use of oral contraceptives (OCP), smoking, and infection with other sexually transmitted diseases (20). Prolonged duration of OCP use is thought to function as a promoter of HPV-related carcinogenesis, not as a facilitator of HPV infection, although the mechanisms are uncertain (21). Tobacco carcinogens have been found in cervical secretions, and it is postulated that smoking constituents may interact with HPV to induce immunologic changes leading to cervical dysplasia, or may produce genomic damage via genotoxins (22,23).
The HPVs are nonenveloped, double-stranded DNA viruses whose circular DNA encodes eight genes, six early genes, which encode nonstructural proteins (E1, E2, E3, E4, E5, and E6) responsible for viral replication and transcription, and two late genes (L1 and L2), which encode the structural components of the viral capsid. HPV can infect the basal cells of the cervical epithelium and replicate in an extrachromosomal form. In HPV-related malignancy, the virus can integrate into cellular DNA and interact with p53 and RB (retinoblastoma), which prolongs the cell cycle, inhibits apoptosis, and can result in malignant transformation (24). Another potential mechanism for carcinogenesis that has been observed in HPV-16–infected cells is aberrant mitotic spindle pole formation resulting in genetic instability (25). The recent production of prophylactic vaccines that induce the generation of neutralizing antibodies to certain oncogenic types of HPV, therefore, represents a major breakthrough in the prevention of cervical cancer.
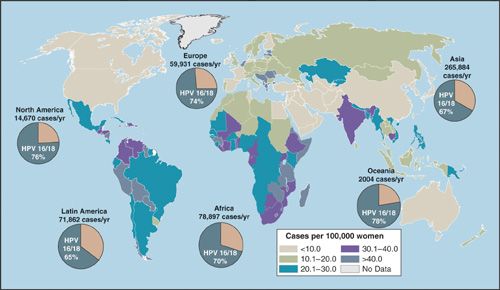
FIGURE 6.1. Age-standardized rates of new cases of cervical cancer per 100,000 women, 2002.
FIGURE 6.2. Human papillomavirus.
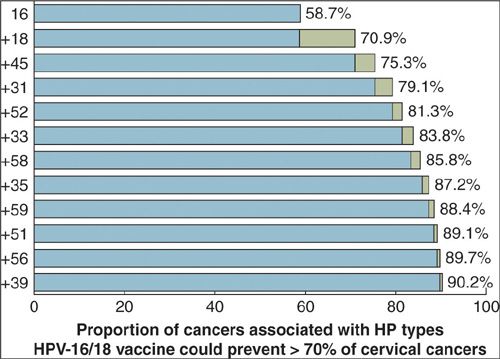
FIGURE 6.3. HPV types in cervical cancer.
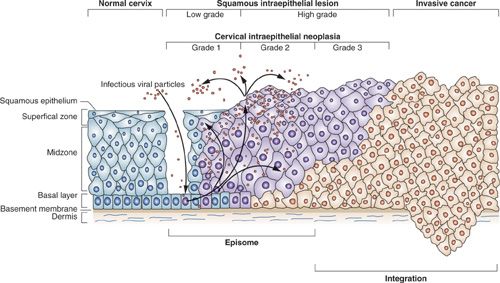
FIGURE 6.4. HPV-mediated progression to cervical cancer.
Prevention
Risk Reduction and/or Adoption of Healthy Practices
Cervical cancer prevention requires decreasing the risk of infection with oncogenic strains of HPV. Having few sexual partners and the use of condoms have been associated with a reduced risk for cervical cancer (26). Avoidance of those factors that enhance the persistence of HPV infection, viz. smoking and OCP use, has the potential to reduce the rate of malignant change. OCPs, however, are the most effective means of contraception, and their avoidance overall is not a wise public health strategy. Safe and effective vaccines now offer the best option for cervical cancer prevention. Early studies in animal models provided the proof of principle that neutralizing antibodies, directed to determinants on the major viral capsid protein, were generated by infection with HPV and could be detected in the serum. In the early 1990s it was found that the L1 protein, when expressed in recombinant vectors, self-assembled into virus-like particles (VLPs), which closely resemble the antigenic characteristics of the wild-type virions. VLPs formulated on aluminum adjuvants were shown to induce a strong virus-neutralizing antibody response in nonhuman primates (27,28), leading to their development for human populations. A series of phase 1 trials in humans tested the immunogenicity and safety of monovalent VLP-based vaccines and found that they generated levels of neutralizing antibodies that far exceeded those seen in natural infections, and were sustained at long-term follow-up. The predominant antibody responses are of the immunoglobulin G1 (IgG1) subclass (18). In these early trials, vaccine efficacy against infection with HPV-16/18 and against CIN 2+ at 6.4 years of follow-up was 100% (29).
Subsequently, 2 vaccines have been developed for use in humans, Gardasil (Merck), a quadrivalent vaccine that includes HPV-16, -18, -6, and -11 and is formulated with aluminum adjuvant, and a bivalent vaccine Cervarix (GlaxoSmithKline), which includes HPV-16 and -18 and is formulated with a proprietary adjuvant, AS04, which contains aluminum and a bacterial lipid. Gardasil has undergone several randomized, placebo-controlled trials among over 21,000 women. In a U.S. multicenter proof of principle study, 2,391 young women aged 16 to 25 were assigned to a monovalent yeast-derived HPV-16L1 VLP, formulated on aluminum adjuvant, by intramuscular injection at day 0, month 2, and month 6 or placebo. The primary outcomes were persistent HPV-16 infection and HPV-16–related carcinoma in situ CIN 2 and 3. At a follow-up of 48 months, administration of the three-dose regimen of HPV-16 vaccine resulted in a 94% reduction in persistent HPV infection in those treated according to protocol. The vaccine was 100% effective in protecting against HPV-16–related CIN 2 and 3 (30).
A phase 2 dose-ranging assessment of immunogenicity and efficacy was conducted in the United States, Brazil, and Europe among 552 women aged 16 to 23 years. Women were randomized to 1 of 3 vaccine doses versus placebo given at day 1, month 2, and month 6. At close to 3 years of follow-up, vaccine efficacy for reduction of persistent HPV infection was 90% and for clinical disease (cytologic abnormalities, CIN, cervical cancer, or external genital lesions) was 100%. Vaccine efficacy was similar for each of the three doses. All women who received the vaccine developed high antibody titers by month 7 (31). Early data on vaccine efficacy found similar results with 2 versus 3 doses, which if borne out, is likely to improve compliance (32).
The two pivotal phase 3 trials enrolled more than 18,000 women and included both precancerous lesions, CIN 2, CIN 3, adenocarcinoma in situ (AIS), or invasive cervical cancer with documented HPV-16 or -18 in DNA from the involved tissue, vulvar and vaginal lesions, and genital warts as outcomes. Women with evidence of previous infection with HPV, or with cytologic abnormalities, were not excluded. At 3 years, vaccine efficacy in those women treated according to protocol was 99%, with only one case of CIN 3 occurring among the vaccine recipients. In the intent to treat population, which included women with prevalent HPV-16/18 infection and women who did not complete the vaccination schedule, CIN 2/3 and AIS were reduced by 44% (33,34). The vaccine was also efficacious against high-grade vulval and vaginal lesions (35). To investigate the efficacy of Gardasil among older women, 3,891 women aged 24 to 45 years were randomized to vaccine or placebo. Efficacy against persistent infection and cervical lesions was 89% (36). Side effects in all of the studies were minimal and included discomfort at the injection site and occasional mild fever. Anti-HPV antibody titers remain high 5 years after administration. In June 2006, Gardasil received FDA approval for the vaccination of women aged 9 to 26, followed closely by approval for its use in children and adults aged 9 to 26 years by the European Commission.
The initial trial of Cervarix randomized 1,113 women aged 15 to 25 years to receive a bivalent HPV-16, -18 vaccine versus placebo. No cases of persistent HPV-16 or HPV-18 were seen in the women vaccinated according to protocol, whereas there were seven cases of infection in the control group. The vaccine was 95% effective against persistent infection and 93% effective against cytologic abnormalities even when including those women who did not complete the vaccine regimen. In an extended follow-up of 776 women, the vaccine continued to demonstrate 98% efficacy at close to 4 years (37,38). The Papilloma TRIal against Cancer In young Adults (PATRICIA) randomized 18,644 women to 3 doses of Cervarix versus hepatitis A vaccine. At a mean of 34.9 months follow-up, HPV vaccine efficacy, assessed in women who were seronegative DNA for HPV-16/18 at baseline, against CIN 2+ lesions was 92% (39).
There was also efficacy against CIN 3+, especially in the 15- to 17-year age group (40). Furthermore, the vaccine provided cross-protective efficacy against 4 oncogenic nonvaccine types: HPV-33, HPV-31, HPV-45, and HPV-51 (41).
Overall, achieving close to 100% effective vaccination of a cohort of 12-year-old girls is estimated to result in a 76% reduction in the incidence of cervical cancer (42). Mathematical modeling has been used to estimate the clinical benefits and cost-effectiveness of a population-based HPV-16/18 vaccination program in Africa (Fig. 6.5). Parameters considered were the natural history of HPV and cervical cancer, age at vaccination, vaccine cost, vaccine efficacy, waning immunity, the risk of replacement with other oncogenic strains of HPV, age at onset of screening, and screening intervals. A review of 27 studies of cost-effectiveness models found considerable variability in the incremental cost-effectiveness ratios (ICER) due to the different assumptions made. Overall, however, the implementation of routine vaccination programs for adolescent girls is consistently shown to be cost-effective compared to screening alone (43). The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends routine vaccination for HPV of girls age 11 to 12 (range, 9 to 26 years) and has added Gardasil to its Vaccines for Children program (44,45). Similarly, the American Academy of Pediatrics recommends that all girls should be vaccinated against HPV at age 11 to 12 years (46).
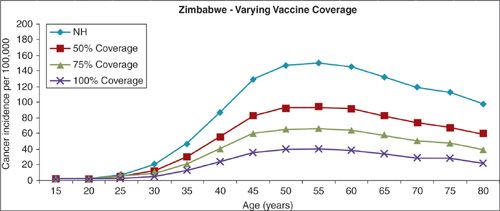
FIGURE 6.5. Impact of HPV-16/18 vaccines on incidence of cer vical cancer.
Both Gardisil and Cevarix are licensed for use in the United States for girls aged 11 to 12 years, with catch up vaccination up to age 26 years. Immunogenicity of both vaccines is close to 100% (47).
There are several questions remaining regarding the use of HPV vaccines. The duration of protection afforded by HPV vaccines is not known, although data from animal models report long-lasting protection even with low levels of circulating antibody. Evidence from recent clinical trials in humans suggests persistence of immunity beyond 4 years. The need for a booster dose is at this time unknown. Because the neutralizing antibodies generated by the current vaccines appear to be type specific, it is not clear if there is cross-reactivity with other HPV types. It is also unclear whether vaccinating an adolescent population will be socially acceptable in all cultures. Most of the randomized trials performed to date focused on young women in their late teens and early 20s, an age when sexual activity has likely already begun. Because HPV vaccines are ineffective after infection with HPV, vaccination should occur prior to the onset of sexual activity, or at a younger age. The majority of females will become infected with HPV within 2 to 3 years of the onset of sexual activity. Depending on socio-cultural variations in sexual debut, the target age for vaccination would ideally be girls aged 9 to 12 years. There is currently no systematic vaccination program beyond infancy and preschool in the United States. Although vaccinating older women who are uninfected with HPV has been shown to induce an effective immune response (36), studies have shown that antibody response is higher among younger age groups (30,48).
Another unanswered question is the added benefit of vaccinating males. Although the majority of morbidity associated with HPV is seen in females, males remain the major vector for the disease. Furthermore, both males and females would need to be vaccinated to achieve herd immunity. Information on the natural history of HPV in males is lacking, and vaccination studies to date have focused predominantly on females. One Phase III study of Gardasil in men reported a 90% reduction in HPV-associated external genital lesions (32). Among women, the impact of effective vaccination on subsequent screening practices is not known. There is concern that women who receive the HPV vaccine might be less likely to adhere to screening guidelines because they feel protected from cervical cancer. Women who have been vaccinated will, however, need to continue cytologic screening since the current vaccines do not protect against all oncogenic types, and since some women will have already been infected at the time of vaccination. It is likely, however, that the onset of screening could safely be delayed. Concerns about the impact of HPV vaccination on the subsequent accuracy of cytologic screening have also been raised.
Much work remains to be done to educate the public about HPV and cervical cancer. Recent studies have shown that the majority of women are unaware of the link between HPV and cervical cancer. Awareness of HPV is increased among young women, more educated women, and those with more access to the health care system (49,50). Public health efforts to introduce the vaccine will clearly need to be accompanied by vigorous educational programs directed at both young women and their parents to increase acceptability and the success of the HPV vaccine program. Since the introduction of Gardasil into clinical care, there has been vigorous debate about the issue of compulsory HPV vaccination. Concerns raised include the lack of long-term safety data, the expense of the vaccine, and resistance to governmental coercion (51). Another concern that parent and other groups have expressed is the fear that vaccination against HPV may lead to a sense of invulnerability, would undermine abstinence-based messages, and may increase high-risk sexual behavior. There is no data, however, to suggest that fear of HPV is an important deterrent from sexual activity in young men or women. Several states have considered legislation to mandate HPV vaccination, although few have actually enacted such laws. All of the proposed laws have opt-out provisions for parents who object. However, they do not address the potential financial burdens imposed by the mandate. Mandating HPV vaccination would certainly boost vaccine coverage rates, but at a price of loss of parental autonomy.
These and other vaccine-related concerns will need to be addressed by primary care providers as well as public health officials. Vaccine delivery by primary care practitioners in the United States is approximately 32% among 13- to 17-year-old girls. In contrast, rates of vaccination completion in the UK and Australia, where vaccine programs are school-based, are much higher (≥84% and ≥72%, respectively) (52).
The most significant unresolved issue pertains to the application of HPV vaccines to underdeveloped nations, where the greatest burden of disease attributable to HPV is found (Fig. 6.5). Contributing to this burden is a lack of understanding of the dimensions of the disease, weak infrastructures and insufficient funds for population-wide vaccination programs, and a lack of the political will to address a sexually transmitted disease. The delivery of a new vaccine to a nonpediatric population is particularly problematic in countries with limited public health resources. Yet it is precisely in these countries that the potential benefit for a widespread vaccination program is greatest. Administering the vaccine in infancy along with other basic childhood vaccines may be the best choice, even though the duration of protection is at this time unknown. Clearly, the contribution of the international community will be required to make HPV vaccination a reality in the third world.
Chemoprevention
Several promising targets for the chemoprevention of cervical cancer have been identified, including topical retinoids, carotenoids, prostaglandins, indole-3-carbinol, green tea, and immune modulators (53,54). In phase 1 and 2 trials, topical retinoids applied directly to the cervix resulted in significant complete histologic regression of CIN 2 lesions compared to placebo (55). None of the proposed agents, however, have been subject to definitive phase 3 randomized trials.
Surgical Prophylaxis
The introduction of widespread cervical cancer screening using the Papaniculaou (Pap) smear has dramatically reduced the incidence of invasive cervical cancer through the detection of treatable, premalignant lesions, referred to as cervical intraepithelial neoplasia (CIN). Recently, the detection and quantification of oncogenic HPV DNA in cervical epithelial cells has been added to routine Pap screening in selected situations, with demonstrated improvement in sensitivity. Cotesting with HPV DNA detects high-grade lesions earlier, thus providing a subsequent longer low-risk period (56). HPV screening is recommended in women with atypical squamous cells of undetermined significance (ASCUS) on cytology, cotesting with cytology in asymptomatic women aged 30 and older, among whom a positive HPV screen is thought to represent persistent infection, and in follow-up of treated individuals for more aggressive detection and management of persistent HPV infection (19,57). Its use in asymptomatic women under the age of 30 leads to overdiagnosis because of the transient nature of infection in this age group. Studies of cost-effectiveness of cotesting with HPV DNA and cytology offer a cost saving by allowing for a reduction in the frequency of screening (58).
Transient HPV infections are associated with low-grade lesions (CIN 1). When oncogenic HPV infections persist, the viral genome is integrated into the host genome and cervical lesions progress to more advanced lesions (CIN 2 and VIN 3) (57). Because of the high rate of spontaneous regression, management of women with CIN 1 and satisfactory colposcopy (visualization of the entire squamocolumnar junction) is repeat cytology at 6 and 12 months or DNA testing for oncogenic types of HPV at 12 months. Alternatively, ablative (cryotherapy, electrocoagulation, or laser vaporization) or excisional (cold-knife conization or loop electrosurgical excision procedure [LEEP]) treatment may be offered. If the entire squamocolumnar junction cannot be visualized, an excisional procedure is the preferred approach. Treatment of CIN 2-3 lesions with satisfactory colposcopy involves excision or ablation of the entire transformation zone rather than just the colposcopically identified lesion (59). Cryotherapy, laser vaporization, and LEEP all appear to be effective modalities, although over time LEEP has become the procedure most widely chosen. When colposcopy is not satisfactory, a diagnostic excisional procedure is recommended. A variety of posttreatment surveillance protocols utilizing cervical cytology with or without colposcopy and HPV testing at frequent intervals have been proposed. Hysterectomy is reserved for recurrent or persistent biopsy-confirmed CIN 2-3, for positive margins when repeat diagnostic excision is not possible, or for women with persistent CIS who have been previously treated and who no longer desire fertility (60). This approach to cervical cancer prevention based on large-scale cytologic screening programs is not feasible, however, in countries in the developing world, due to lack of infrastructure, funding, and public health education (Tables 6.1 and 6.2).
Cervix Cancer—Major Points |
Virtually all cervix cancer is related to persistent infection with HPV
HPV infection is common, affects up to 50% of women, and peaks in the second and third decades of life
Altered immune status, smoking, and the use of OCPs affect the rate of persistent HPV infection
Cervical cancer is a leading cause of cancer morbidity and mortality among women in the underdeveloped world
Two HPV vaccines have shown high efficacy in eliminating persistent HPV infection and cervical lesions in previously uninfected women
HPV, human papillomavirus; OCPs, oral contraceptives.
Cervix Cancer—Remaining Questions |
What is the duration of protection of HPV vaccines?
What is the extent of cross-vaccination with the current HPV vaccines?
What is the socio-cultural acceptability of vaccinating adolescent girls?
What is the added benefit of vaccinating males?
What is the impact of effective vaccination on subsequent screening practices and screening performance?
Which methods will best educate the public about HPV vaccines?
How should HPV vaccines be made available to underdeveloped nations?
What are the best cervical cancer screening approaches for use in the underdeveloped countries?
HPV, human papillomavirus.
OVARIAN CANCER
Risk Factors
Ovarian cancer is the most common cause of death from a gynecologic cancer in the United States and accounts for approximately 15,500 deaths per year (16). Worldwide there are 192,000 new cases per year. Ovarian cancers are categorized as serous, endometriod, clear cell, and mucinous. These categories are further divided into high-grade and low-grade. High-grade serous cancer represents the majority of epithelial ovarian cancer (70%) (61). Due to a lack of effective screening tools to identify ovarian cancer at early, highly curable stages, the majority of ovarian cancers are diagnosed at advanced stages when survival is poor. Historically, the origin of epithelial ovarian cancer was believed to be from the invagination of ovarian surface epithelium (OSE) into the ovarian stroma forming inclusion cysts, which had the potential to undergo malignant transformation (62). The recent adoption of prophylactic salpingo-oophorectomy for women with deleterious mutations in BRCA1 and BRCA2, however, has challenged that theory. Careful examination of the fallopian tubes obtained at the time of prophylactic surgery has identified a high prevalence of occult primary serous carcinomas and serous tubal intraepithelial carcinomas (STIC) in the fimbrial end of the fallopian tube. Both lesions are often accompanied by p53 mutations, suggesting that STIC is the precursor lesion for invasive ovarian carcinoma. While these changes were originally thought to be present only in women with BRCA1 or BRCA2 mutations, there is growing evidence pointing to the existence of these precursor lesions among women who develop sporadic serous ovarian cancer (63). The distal fallopian tube, therefore, is increasingly being seen as the origin of tubal, ovarian, and peritoneal serous ovarian cancer. The fallopian tube is also implicated in the presentation of endometrioid and clear cell ovarian cancers, which are attributed to the passage of endometriosis tissue from the uterus through the fallopian tube to implant on the surface of the ovary or the peritoneum where it can undergo malignant transformation (64).
While our understanding of the biology of epithelial ovarian cancer is rapidly advancing, most of our current knowledge regarding risks for ovarian cancer has emerged from the epidemiologic literature. Advancing age, reproductive factors (specifically, nulliparity), and heredity are established risk factors for the disease. The majority of ovarian cancers are diagnosed after menopause. Rates are higher in nulliparous women, while parity has been found to offer protection. The risk of ovarian cancer is increased twofold in women who are infertile, and the risk appears to be independent of fertility drug treatment (65). Chronic inflammation, with its attendant increase in cell proliferation and potential for DNA disruption, has been proposed as a precursor for many cancers, including ovarian cancer. Endometriosis and pelvic inflammatory disease, both of which induce chronic inflammatory states, are associated with ovarian cancer (64).
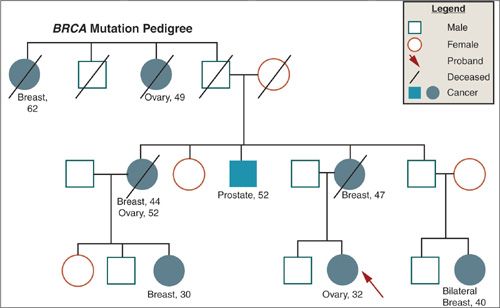
Although the majority of cases of ovarian cancer are sporadic, approximately 5% to 10% are thought to fit a hereditary pattern of autosomal dominant inheritance. Epidemiologic studies have estimated a two- to fourfold increase in risk among first-degree relatives of women with ovarian cancer. Recently, a number of genes have been identified that account for a large percentage of hereditary ovarian cancer and that allow more precise estimates of risk. Since the identification of BRCA1 on chromosome 17q in 1994, and BRCA2 on chromosome 13q in 1995, several hundred mutations in these genes have been characterized, many of which lead to premature truncation of protein transcription and, therefore, presumably defective gene products. Ovarian cancer in these families is characterized by multiple cases of ovarian and breast cancer in successive generations, earlier age of onset, and evidence of both maternal and paternal transmission (Fig. 6.6). The penetrance of BRCA1/2, that is, the likelihood that a mutation will actually result in ovarian cancer, is estimated to range anywhere from 36% to 46% for BRCA1 mutation carriers, and from 10% to 27% for BRCA2 mutation carriers (66). Some mutations may be more specifically related to ovarian cancer risk than others. An ovarian cancer cluster region has been identified, for example, in exon 11 of BRCA2, which is associated with a higher rate of ovarian cancer than other mutations in the gene. The wide variation in penetrance observed may also reflect the interaction of the genetic mutation with other genetic and/or environmental factors, and suggests that these genes may function as “gatekeepers” and, when lost, allow other genetic alterations to accumulate. Ovarian cancer is also included in the phenotype of the recently described mismatch repair genes associated with the hereditary nonpolyposis colon cancer (HNPCC), or Lynch syndrome, in which the lifetime risk for ovarian cancer is estimated to be approximately 12%, and the median age at diagnosis 42.7 years (67) (Fig. 6.7). The identification of hereditary syndromes of ovarian cancer provides new opportunities to understand the biology of the disease and to devise novel preventive strategies.
FIGURE 6.6. Family pedigree illustrating BRCA mutation.
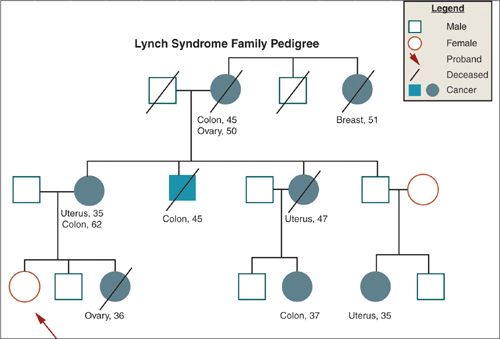
FIGURE 6.7. Family pedigree illustrating Lynch mutation.
Prevention
Risk Avoidance and/or Adoption of Protective Practices
The available evidence regarding factors that lower ovarian cancer risk has been based primarily on the results of case-control studies retrospectively comparing the reproductive, hormonal, or behavioral characteristics of ovarian cancer cases with matched controls, not on prospective randomized trials. These studies have consistently shown an inverse association of ovarian cancer with increasing parity, with the first birth conferring significantly more protection (35%) than subsequent births (15%). The protective effect of pregnancy occurs regardless of fertility history and is not age dependent (68). Pregnancy is characterized by a prolonged period of anovulation as well as high levels of circulating progesterone, which may cause terminal differentiation of premalignant cells (69,70).
The evidence supporting a protective effect of breast-feeding against epithelial ovarian cancer risk is weak and inconsistent. Some studies suggest a 10% to 20% decrease in ovarian cancer risk associated with breast-feeding. In those studies that are positive, the impact of breast-feeding on ovarian cancer risk appears to be greatest for the first 6 months of lactation with no apparent increase in ovarian cancer protection with longer-term lactation (71,72).
Several studies have examined the association of hormone replacement therapy (HRT) with ovarian cancer risk. While the majority find a modest increase in risk, most lack statistical significance. The strongest association is seen in endometrioid histologies, where relative risks range from 1.2 to 5.5 (73).
Several retrospective and prospective studies have examined associations between dietary factors and ovarian cancer risk. Modest levels of protection have been reported for fruits and vegetables in general, and for vitamin A and β-carotene in particular, although findings are inconsistent (74,75). While there is some case-control data that invasive ovarian cancer is reduced among women who report frequent high-intensity exercise, the relationship between physical activity and ovarian cancer risk is inconsistent, and may differ by histologic type (65,76,77).
Chemoprevention
The majority of theoretical models of ovarian cancer chemoprevention have been based on the assumption that the origin of ovarian cancer is the OSE. As the paradigm has shifted to identifying the epithelial surface of the fallopian tube as the site of origin, it is not clear how to interpret the previous theories. Prior research has explored the presence of receptors for most members of the steroid hormone superfamily, including receptors for progestins, retinoids, androgens, and vitamin D. Progestins, retinoids, and vitamin D have been shown to exert a broad range of common biologic effects in epithelial cells, including induction of apoptosis, upregulation of transforming growth factor-β (TGF-β), cellular differentiation, and inhibition of proliferation. In addition to hormonal agents, there is growing evidence that nonsteriodal anti-inflammatory drugs (NSAIDs) may have ovarian cancer preventive effects (78). It is yet to be demonstrated that these agents are active in the fallopian tube epithelium.
To date, only OCP use has been consistently shown to be protective against ovarian cancer. OCPs were first introduced in the United States in the 1960s. Most formulations include estrogen, progesterone, or a combination of the 2. In addition to suppressing ovulation, OCPs also reduce pituitary secretion of gonadotropins and protect against chronic inflammation associated with pelvic inflammatory disease (79). In addition to these potential mechanisms, a 3-year study on primates demonstrated that the progestin component of an OCP had a potent effect on apoptotic and TGF-β signaling pathways in the ovarian epithelium, raising the possibility that progestin-mediated biologic effects may underlie the protective effects of OCPs (80). The use of OCPs appears to decrease a woman’s risk for ovarian cancer by 30% to 60%. Risk reduction is apparent with as little as 3 months of use, increases in magnitude with increased duration of use, and persists for as long as 10 years after discontinuation of use. The risk reduction applies to nulliparous as well as parous women, to all histologic subtypes, including tumors of low malignant potential, to women with a hereditary risk for ovarian cancer, is consistent across races, and is independent of age at use or menopausal status (70,81,82). Although there has never been a randomized clinical trial to demonstrate the protective effect of OCPs on ovarian cancer risk, it is often recommended empirically to women with a family history of ovarian cancer to reduce their risk.
Epidemiologic and laboratory evidence suggest a potential role for retinoids as preventive agents for ovarian cancer (83). Retinoids are natural and synthetic derivatives of vitamin A. They have great potential for cancer prevention, due to a broad range of important biologic effects on epithelial cells, including inhibition of cellular proliferation, induction of cellular differentiation, induction of apoptosis, cytostatic activity, and induction of TGF-β. The most significant evidence supporting a rationale for retinoids as chemopreventive agents for ovarian cancer is that of a chemoprevention study in Italy, which suggested an ovarian cancer preventive effect from the retinoid 4-HPR. Among women randomized to receive either 4-HPR or placebo in a trial designed to evaluate 4-HPR as a chemopreventive for breast carcinoma, significantly fewer ovarian cancer cases were noted in the 4-HPR group as compared to controls (84).
Vitamin D is a fat-soluble vitamin that is essential as a positive regulator of calcium homeostasis. The vitamin D receptor and the retinoic acid receptors share strong homology and readily dimerize, making it likely that vitamin D and retinoids have common signaling pathways in the cell (85). Vitamin D has been shown to have diverse biologic effects in epithelial cells relevant to cancer prevention, including retardation of growth, induction of cellular differentiation, induction of apoptosis and upregulation of TGF-β (86). With regard to ovarian cancer, a recent study has correlated population-based data regarding ovarian cancer mortality in large cities across the United States with geographically based long-term sunlight data reported by the National Oceanic and Atmospheric Administration. The study demonstrated a statistically significant inverse correlation between regional sunlight exposure and ovarian mortality risk suggesting that sunlight induces production of native vitamin D in the skin (87). However, a systematic review of 20 ecologic and case-control studies failed to find consistent evidence for a relationship between vitamin D exposure and ovarian cancer incidence or mortality (88).
Epidemiologic studies have suggested that use of NSAIDs may lower ovarian cancer risk (89). Several biologic mechanisms have been proposed to account for the chemopreventive effects of NSAIDs, including inhibition of ovulation, inhibition of COX, and downregulation of prostaglandins, enhancement of the immune response, and induction of apoptosis (70,90,91). Similarly, dietary antioxidants have shown an inverse association with ovarian cancer (92). Despite a growing body of preclinical data indicating chemopreventive effects of several agents, clinical research exploring their efficacy to reduce rates of ovarian cancer is hindered by the relatively low incidence of the disease, insufficient understanding of the preclinical course of ovarian cancer, the lack of validated preclinical biomarkers, and inadequate screening strategies.
Surgical Prophylaxis
For women with a family history of ovarian cancer, or a hereditary pattern of breast cancer, bilateral salpingo-oophorectomy (BSO) has been shown to lower the risk of subsequent epithelial ovarian cancer by 80% to 95% (66,93). Prospective follow-up of a large international cohort of 2,482 BRCA carriers found not only a lower risk of ovarian cancer, but also a significantly lower all-cause mortality (HR 0.40; 95% CI, 0.26–0.61) and ovarian cancer specific mortality (HR 0.21; 95% CI, 0.06–0.76). There is also a 50% reduction in rates of breast cancer in women who undergo prophylactic BSO (94). Occult invasive and in situ tumors have been found at the time of BSO in 2% to 10% of BRCA1/2 mutation carriers (95), a large proportion of which occur in the fimbrial end of the fallopian tube (79,96), emphasizing the need for both deliberate removal of the fallopian tubes at the time of prophylactic surgery, and of careful pathologic examination of the surgical specimen (97). The incidence of primary peritoneal cancer following BSO is reported to be approximately 2% to 5% (98). Because the median age of diagnosis of ovarian cancer among women with a hereditary risk is 50 years, the recommended age for prophylactic surgery is at the completion of childbearing, or at age 35 to 40 years. Although the incidence of premenopausal ovarian cancer is higher in BRCA1 carriers than BRCA2 carriers, removal of the ovaries before menopause is recommended for both groups given the added benefit of breast cancer risk reduction, which is highest for women who undergo the surgery before natural menopause (17). In addition, because there is an approximate 15% risk of ovarian cancer after age 60 years among BRCA carriers, BSO is also justified at older ages for women who still have intact ovaries (99).
Women undergoing prophylactic hysterectomy for a deleterious mutation related to Lynch syndrome are also counseled to remove their ovaries because of the approximate 10% to 12% incidence of ovarian cancer (100).
In addition to the significant reduction in ovarian cancer incidence associated with BSO, several studies have found a significant decline in ovarian cancer worry and anxiety following the procedure (101). These potential benefits of prophylactic oophorectomy must, however, be weighed against its adverse consequences, including the short- and long-term surgical risks, the physical and psychological impact of early menopause, and the potential subsequent risks of cardiovascular disease and osteoporosis related to early estrogen/progesterone depletion (102). Although the use of combined estrogen/progesterone HRT has been associated with an increased risk for breast cancer among postmenopausal women in the general population, one study with short follow-up found no increased risk of breast cancer among BRCA1/2 mutation carriers who took HRT following BSO (11). Also, data from the Mayo Clinic showed that there was no increase in breast cancer among women under the age of 50 years undergoing BSO (103). Women seeking information regarding prophylactic oophorectomy should be counseled about the practical short- and long-term sequelae of the surgery, the risks and benefits of postoperative HRT, and the small potential for primary peritoneal cancer.
Given the recent evidence that a majority of ovarian cancers arise in the fimbrial end of the fallopian tube, an alternative surgical procedure, radical fimbriectomy, has been introduced. The principle is that complete removal of both fallopian tubes will remove the source of the premalignant changes in the fallopian tube, which give rise to serous ovarian cancer. In addition, removing the tubes will prevent any endometrial tissue from reaching the ovary or peritoneal cavity. Because prospective data to establish the efficacy of this approach are lacking, and because there is still a proportion of ovarian cancer that may arise out of inclusion cysts in the ovary, radical fimbriectomy is currently proposed as a temporary solution which will prolong the production of ovarian hormone for BRCA1/2 carriers, thus postponing the onset of premature menopause (104,105).
Ovarian Cancer—Major Points |
Several risk factors, including age, nulliparity, and family history, have been identified for ovarian cancer
Germ-line mutations in the BRCA1/2 genes and the DNA mismatch repair genes associated with Lynch syndrome significantly increase the risk of ovarian cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree