Armando E. Giuliano
Breast cancer accounts for approximately one-third of all cancers in women and is second only to lung cancer as the leading cause of cancer deaths among women. Breast cancer has the highest incidence rate of all cancers. According to statistics from the American Cancer Society (ACS), over 230,000 new cases of invasive breast cancer will be diagnosed in women during 2011 in the United States as well as over 55,000 patients of in situ cancers, with almost 40,000 women succumbing to the disease during the same period (1). Over the past 50 years, the incidence of breast cancer in the United States increased significantly; one in every seven women will develop the disease during her lifetime. Fortunately, the mortality rate has declined since 1990.
Predisposing Factors
Less than 1% of breast cancers occur in women younger than 25 years of age. After age 30, there is a sharp increase in the incidence of breast cancer. Except for a short plateau between the ages of 45 and 50 years, the incidence increases steadily with age (2).
Family History
Of women who develop breast cancer, 20% to 30% have a family history of the disease. Although any family history of breast cancer increases the overall relative risk, this risk is not significantly increased if the disease was diagnosed postmenopausally in a first-degree or more distant relative (3). If a woman’s mother or sister had unilateral breast cancer premenopausally, her lifetime risk of developing the disease approaches 30%, whereas a woman whose mother or sister had bilateral breast cancers premenopausally has at least a 40% to 50% lifetime risk. The increased incidence in these women is probably the result of inherited oncogenes.
Approximately 5% to 10% of breast cancers have an inherited basis. All inherited genes are autosomal dominant but have variable penetrance. Men carry the gene 50% of the time. The most common mutations are the BRCA1 (chromosome 17q21) and BRCA2 (chromosome 13q12-13) gene deletions. Carriers of these germline mutations have up to a 4% per year risk of developing breast cancer and a lifetime risk that ranges from 35% to 85% (4). These individuals have up to a 65% risk of developing a contralateral breast cancer. The BRCA1 mutation is associated with an increased risk of ovarian and prostate cancer, whereas BRCA2 carriers, although less common, demonstrate increased risks of male breast and prostate cancers. Both mutations are rare in the general public (0.1%) but are more commonly identified in Jews of Ashkenazi descent (1% to 2.3%) (5). Genetic testing is available and should be considered if there is a high likelihood that results will be positive and will be used to influence decisions regarding the clinical management of the care of the patient and her family. Patients with three of more relatives with breast or ovarian cancer with one of the relatives being diagnosed before the age of 50, two first- or second-degree relatives with breast or ovarian cancer, any relative with male breast cancer, patients whose cancer has been diagnosed before the age of 50, and any patients with both breast and ovarian cancer in their family should undergo genetic counseling for BRCA testing. Ashkenazi Jewish patients should undergo genetic counseling if any first-degree relative, or two second-degree relatives on the same side have breast or ovarian cancer (6). Genetic testing is increasingly important given the evidence that prophylactic surgery may prevent new cancers from occurring, as well as prolong survival, in some cases. A prospective multicenter cohort study of 2,482 women with BRCA1 and BRCA2 mutations between 1974 and 2008 showed risk-reducing mastectomy was associated with a decreased risk of breast cancer. Risk-reducing salpingo-oophorectomy was associated with decreased risk of ovarian cancer, first diagnosis of breast cancer, all-cause mortality, breast cancer–specific mortality, and ovarian cancer–specific mortality (7).
Diet, Obesity, and Alcohol
There are marked geographic differences in the incidence of breast cancer that may be related to diet. A meta-analysis demonstrated an association between a healthy diet and lower risk of breast cancer (8). Although a definitive relationship between total alcohol consumption and increased risk of breast cancer has yet to be determined, high wine intake was associated with elevated risk (8,9).
Reproductive and Hormonal Factors
The risk of breast cancer increases with the length of a woman’s reproductive phase (10). Although early menarche was reported among breast cancer patients, early menopause appears to protect against the development of the disease, with artificial menopause from oophorectomy lowering the risk more than early natural menopause (11). There is no clear association between the risk of breast cancer and menstrual irregularity or the duration of menses. Although lactation does not affect the incidence of breast cancer, women who were never pregnant have a higher risk of breast cancer than those who are multiparous. Women who give birth to their first child later in life have a higher incidence of breast cancer than do younger primigravida women (12).
A historic well-controlled study from the Centers for Disease Control and Prevention showed that oral contraceptive use does not increase the risk of breast cancer, regardless of duration of use, family history, or coexistence of benign breast disease (13). A pooled analysis from 54 epidemiologic studies showed current users of oral contraceptives had a small but significant increased risk when compared with nonusers. Ten years after discontinuation, the risk of past users declined to that of the normal population (14).
It was reported that short-term estrogen treatment for menopausal symptoms did not increase the risk of breast cancer, but this belief was refuted by the results of the Women’s Health Initiative randomized trial. This prospective trial, involving 16,000 postmenopausal women randomly assigned to receive estrogen plus progesterone or placebo, revealed an association between hormone therapy use and the development of breast cancer. When invasive breast cancer developed, it was diagnosed at a more advanced stage compared with tumors that developed among placebo users. Based on interim analysis, the trial was stopped early and the investigators concluded that even relatively short-term use of combined estrogen–progesterone therapy increases the development of invasive breast cancer (15). The risk demonstrated by this study must be considered when postmenopausal hormone therapy is used to treat conditions such as hot flashes and osteoporosis.
History of Cancer
Women with a history of breast cancer have a 50% risk of developing microscopic cancer and a 20% to 25% risk of developing clinically apparent cancer in the contralateral breast, which occurs at a rate of 1% to 2% per year (16). Lobular carcinoma has a higher incidence of bilaterality than does ductal carcinoma. A history of endometrial, ovarian, or colon cancer is associated with an increased risk of subsequent breast cancer, as is a history of radiation therapy for Hodgkin’s lymphoma, even if the patient is BRCA1 and BRCA2 negative.
Diagnosis
Breast cancer commonly arises in the upper outer quadrant, where there is proportionally more breast tissue. Masses are often discovered by the patient and less frequently by the physician during routine breast examination. The increasing use of screening mammography has enhanced the ability to detect nonpalpable breast abnormalities. Metastatic breast cancer is found as an axillary mass without obvious malignancy in less the 1% of cases.
The standard screening modalities of mammography and physical examination are complementary. Approximately 10% to 50% of cancers detected mammographically are not palpable, whereas physical examination detects 10% to 20% of cancers not seen radiographically (17). The purpose of screening is to detect tumors when they are small (<1 cm) and have the highest potential for surgical cure. Most trials show a 20% to 30% reduction in breast cancer mortality for women age 50 and older who undergo annual screening mammography. Data on screening women younger than 40 years are more controversial. Results from the Gothenburg screening trial showed a 45% reduction in mortality for women screened between the ages of 40 and 49 (18). Because of these findings, it is recommended that all women undergo yearly screening mammography starting at age 40, along with clinical breast examination at least every 3 years (19). Monthly breast self-examination (BSE) is no longer recommended because there is little evidence to show that BSE is superior to heightened breast awareness such that any new symptoms related to the breast in daily activities would be reported promptly when noticed. BSE was not shown to improve survival. Women should be informed about the benefits and limitations of monthly BSE, mainly the risk of a false-positive result. Women who would still prefer to perform BSE should be instructed in the technique and occasionally have their technique reviewed. No other tests, including ultrasonography, computed tomography (CT) scans, sestamibi scans, positron emission tomography (PET) scans, or serum blood markers, have been shown to be effective screening modalities. Screening guidelines recommended by the American College of Radiology and the American Cancer Society are presented in Table 40.1. In 2010, the ACS recommended annual screening mammography and magnetic resonance imaging (MRI) starting at age 30 for women with a known BRCA mutation and other high-risk genetic syndromes, women who are untested with a first-degree relative with the BRCA mutation and women with an approximately 20% to 25% or greater lifetime risk of breast cancer, or women who have been treated with radiation for Hodgkin’s lymphoma (19). Although breast MRI may prove to be advantageous for other women with an elevated risk of breast cancer, there is currently insufficient evidence to make recommendations for women with lower than a 20% lifetime risk of breast cancer (19).
Table 40.1 Screening Recommendations
| Bilateral mammograms |
| Beginning at age 40 yearly mammograms, which should continue as long as the patient is in good health. |
| Self-examination |
| Is an option for women starting in their 20s. Women should be counseled on the benefits and limitation of breast self-examination and should be told to report any changes in their breasts to their health professional right away. |
| Clinical breast examination |
| Age 20–40 examination by physician every 3 years, annually if positive history |
| (May do annually if there is a positive family history) |
| Age ≥ 40 examination by physician every year |
| Breast magnetic resonance imaging (MRI) |
| High risk women (greater than 20% lifetime risk) should undergo MRI and mammography every year |
| Medium risk women (15%–20% lifetime risk) should talk to their health care professional about the benefits and limitations of adding MRI to their yearly mammographic screening. |
| Low risk women (less than 15% lifetime risk) are not recommended to undergo additional MRI screening. |
From American Cancer Society Screening Guidelines. Smith RA, Cokkinides V, Brooks D, et al. Cancer screening in the United States, 2010: a review of current American Cancer Society Guidelines and Issues in Cancer Screening. CA Cancer J Clin 2010;60:99–119. |
Masses are easier to palpate in older women with fatty breasts than in younger women with dense, nodular breasts. An area of thickening amid normal nodularity may be the only clue to an underlying malignancy. Skin dimpling, nipple retraction, or skin erosion, while obvious, are later-stage disease signs. Algorithms for the evaluation of breast masses in premenopausal and postmenopausal women are presented in Chapter 21.
When a dominant breast mass is identified, the presence of a carcinoma must be considered, and biopsy should be performed to establish a tissue diagnosis. About 30% to 40% of lesions clinically believed to be malignant will be benign on histologic examination (20). Conversely, 25% of clinically benign-appearing lesions will be malignant when biopsied (21).
Biopsy Techniques
It is preferable for the patient to be involved in planning her therapy. In most instances, initial biopsy can be followed by definitive treatment at a later date. This approach allows the physician to discuss alternative forms of surgical therapy with the patient who has a malignancy. It gives the patient an opportunity to obtain a second opinion before undergoing definitive treatment.
Fine-Needle Aspiration Cytology
Fine-needle aspiration cytology (FNAC) is usually performed on palpable lesions or under ultrasound guidance using a 20- or 22-gauge needle. The technique has a high level of diagnostic accuracy, with low false-negative rates and rare, but persistent, false-positive results (20,22). In most reported series, false-negative rates range from 10% to 15%, and false-positive rates are generally less than 1%, whereas insufficient specimens account for about 15% of samples (23). If a mass appears malignant on physical examination, mammography, or both, FNAC cytology results can be used for definitive diagnosis, although in these circumstances a core biopsy is usually the biopsy of choice. Negative FNAC results do not exclude malignancy and should be evaluated by either a core needle or traditional excisional biopsy for suspicious lesions. In younger women, it is prudent to monitor a benign-appearing mass for one or two menstrual cycles. Confirmation of a clinically apparent fibroadenoma with FNAC can serve as the basis for observational follow-up without excision. FNAC may be used in cystic lesions to aspirate fluid, especially in benign-appearing cystic lesions. For benign cysts, cytologic examination is not necessary if the fluid is nonbloody and the cyst resolves after aspiration.
Core Needle Biopsy
Core needle biopsy (CNB) can be performed on both palpable and nonpalpable breast masses. Performing a core biopsy instead of FNAC on a palpable lesion has the advantage of obtaining more tissue for diagnostic purposes, including tests for estrogen and progesterone receptors and Her-2/neu, and has generally replaced FNAC unless aspiration of a cystic mass is being performed. Core biopsy of nonpalpable breast lesions usually is performed using mammographic or ultrasonographic guidance. MRI-detected lesions that cannot be seen with mammography or ultrasonography may be biopsied under MRI guidance. Mammographic units with computerized stereotactic modifications can be used to localize abnormalities and perform CNB without surgery. Under imaging guidance, a biopsy needle is inserted into the lesion and a core of tissue is removed for histologic examination. Devices with suction assistance often are used to increase the volume of tissue removed for evaluation. A titanium clip often is used to mark the biopsy site and serve as a guide should further excision be required. Ultrasonography may be used to perform core biopsy on a nonpalpable lesion. Because it is less invasive and less expensive than open mammographic localization biopsy, CNB is preferred for accessible lesions. If a definitive diagnosis is not established, these procedures must be followed by open biopsy.
Open Biopsy
Open biopsy may be performed if the lesion cannot be successfully biopsied with a needle, or if FNAC or CNB has shown a lesion that may be associated with malignancy, such as atypical ductal hyperplasia (ADH) or lobular carcinoma in situ (LCIS). Excision must be performed if the results with needle biopsy are equivocal or discordant with the clinical findings. An unequivocal histologic diagnosis of cancer should be obtained before treatment of breast cancer is undertaken. Cytologic diagnosis may be relied on if the mass clinically or mammographically appears to be malignant.
Open biopsy can be performed in the outpatient setting with local anesthesia in the following manner (although this technique largely is replaced by core biopsy and usually is not necessary):
Image-Guided Localization Biopsy
Biopsy of nonpalpable lesions is a potentially difficult procedure that requires close cooperation between the surgeon and radiologist. Using ultrasonographic, mammographic, or MRI guidance, a needle or specialized wire is placed into the breast parenchyma at or near the site of the suspected abnormality. Some mammographers will inject a biologic dye into the breast parenchyma to assist localization. The surgeon reviews the films and localizes the abnormality with respect to the tip of the wire or needle. Alternatively, the surgeon will perform ultrasonography intraoperatively to directly localize the lesion. An incision is made directly over the abnormality, and a small portion of the breast tissue suspected of containing the abnormality is excised. For mammographically detected lesions, a specimen radiograph is obtained to ensure that the abnormality has been recovered. Often, the radiologist can place a needle in the specimen at the site of the abnormality to facilitate histologic evaluation and ensure that the pathologist examines the site of the abnormality. Image-guided biopsy should be performed only for lesions inaccessible to needle biopsy or those lesions that may be associated with malignancy such as ADH.
Pathology and Natural History
Breast cancer may arise in the intermediate-size ducts, terminal ducts, or lobules. In most cases, the diagnosis of lobular and intraductal carcinoma is based more on histologic appearance than site of origin. The cancer may be either in situ (ductal carcinoma in situ or lobular carcinoma in situ) or invasive (infiltrating ductal carcinoma, infiltrating lobular carcinoma). Morphologic subtypes of infiltrating ductal carcinoma include scirrhous, tubular, medullary, and mucinous carcinoma.
True invasive ductal carcinoma accounts for 80% of all invasive tumors, with the final 20% split evenly between lobular carcinoma and special variants of infiltrating ductal carcinoma (24). Mammographically, invasive ductal cancers are characterized by a stellate density or microcalcifications. Macroscopically, gritty, chalky streaks are present within the tumor that most likely represent a desmoplastic response. Invasion of the surrounding stroma and fat, with a fibrotic, desmoplastic reaction surrounding the invasive carcinoma, generally is present.
Special types of infiltrating ductal carcinoma are uncommon and typically account for nearly 10% of all invasive cancers. Medullary carcinoma, which accounts for 5% to 8% of breast carcinomas, arises from larger ducts within the breast and has a dense lymphocytic infiltrate. The tumor appears to be a slower growing and less-aggressive malignancy than other forms of carcinoma. Even when axillary disease is present, the prognosis with medullary carcinoma is better than that of other variants of invasive ductal carcinoma. Mucinous (colloid) carcinoma accounts for 5% of all breast cancers. Grossly, areas of the tumor may appear mucinous or gelatinous, whereas microscopically they are relatively acellular. Infiltrating comedo carcinoma accounts for less than 1% of all breast malignancies and is an invasive cancer characterized by foci of necrosis that exude a comedonecrosis-like substance when biopsied. Usually, comedocarcinomas are in situ malignancies. Papillary carcinoma is predominantly a noninvasive ductal carcinoma; when invasive components are present, it should be specified as invasive papillary carcinoma. Tubular carcinoma, a well-differentiated breast cancer that accounts for 1% to 2% of all malignant breast neoplasms, rarely metastasizes to axillary lymph nodes and tends to have a better prognosis than infiltrating ductal carcinoma. Adenoid cystic carcinomas are extremely rare breast tumors that histologically are similar to those seen in the salivary glands. They are well-differentiated cancers that are slow to metastasize.
Growth Patterns
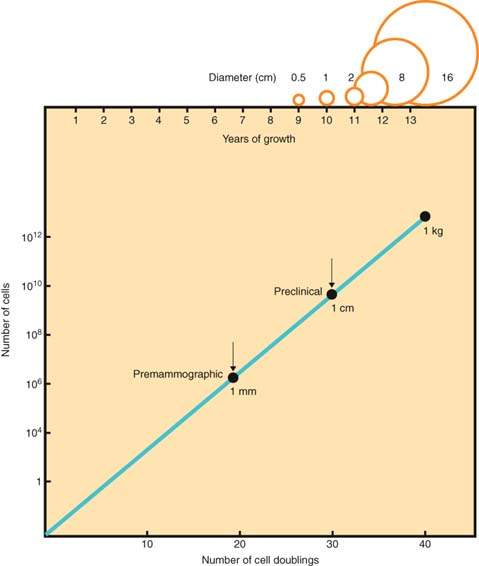
The growth potential of breast cancer and the patient’s resistance to malignancy vary widely with the individual and the stage of disease. The doubling time of breast cancer ranges from several weeks for rapidly growing tumors to months or years for slowly growing lesions. If the doubling time of a breast tumor were constant and a tumor originated from one cell, a doubling time of 100 days would result in a 1-cm tumor in about 8 years (Fig. 40.1) (25). During the preclinical phase, tumor cells may be circulating throughout the body. Because of the long preclinical tumor growth phase and the tendency of infiltrating lesions to metastasize early, many clinicians view breast cancer as a systemic disease at the time of diagnosis. Although cancer cells may be released from the tumor before diagnosis, variations in the tumor’s ability to grow in other organs and the host’s response to tumor cells may inhibit dissemination of the disease. Many women with breast cancer can be treated successfully with surgery alone, and some patients have been cured even in the presence of palpable axillary disease. A pessimistic attitude that breast cancer is systemic and incurable at diagnosis is unwarranted.
Figure 40.1 Growth rate of breast cancer indicating long preclinical phase. (From Gullino PM. Natural history of breast cancer: progression from hyperplasia to neoplasia as predicted by angiogenesis. Cancer 1977;39:2699.)
A more realistic approach may be to view breast cancer as a two-component disease involving the breast and the body as a whole. Although the primary breast tumor and issues of local control must be managed, the possibility of systemic metastases with their life-threatening consequences should not be overlooked.
Breast cancer can metastasize to any organ, and involvement of bone, lungs, or liver occurs in up to 85% of women who develop distant disease (26,27). In addition to these sites, invasive lobular carcinoma is known to disseminate to the abdominal viscera, uterus, ovaries, and peritoneal surfaces.
Staging
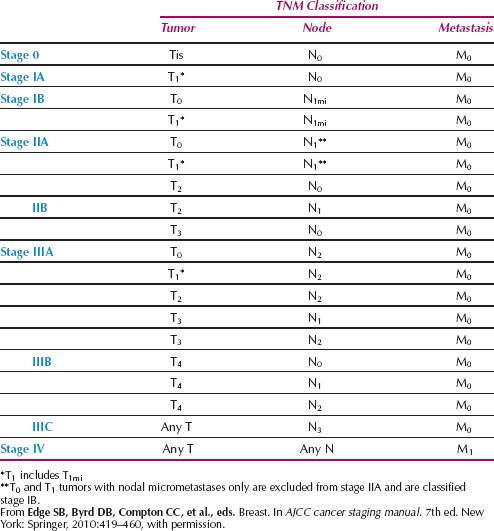
After the diagnosis of breast cancer is definitively established, the clinical stage of the disease should be determined. The Columbia Clinical Staging System was used historically but is replaced by the tumor-node-metastases (TNM) system of the American Joint Committee on Cancer (28,29). The TNM system allows both preoperative clinical staging and postoperative pathologic staging to be determined (Tables 40.2 and 40.3).
Table 40.2 Tumor-Node-Metastasis (TNM) System for Staging of Breast Cancer
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Tis (DCIS) | Ductal carcinoma in situ |
| Tis (LCIS) | Lobular carcinoma in situ |
| Tis (Paget’s) | Paget’s disease of the nipple NOT associated with invasive carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget’s disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget’s disease should still be noted. |
| T1 | Tumor ≤20 mm in greatest dimension |
| T1mi | Tumor ≤1 mm in greatest dimension |
| T1a | Tumor >1 mm but ≤5 mm in greatest dimension |
| T1b | Tumor >5 mm but ≤10 mm in greatest dimension |
| T1c | Tumor >10 mm but ≤20 mm in greatest dimension |
| T2 | Tumor >20 mm but ≤50 mm in greatest dimension |
| T3 | Tumor >50 mm in greatest dimension |
| T4 | Tumor of any size with direct extension to chest wall and/or skin (ulceration or skin nodules) |
| T4a | Extension to chest wall, not including only pectoralis muscle adherence/invasion |
| T4b | Ulceration and/or ipsilateral satellite nodules and/or edema (including peau d’orange) of the skin which do not meet the criteria for inflammatory carcinoma |
| T4c | Both T4a and T4b |
| T4d | Inflammatory carcinoma |
| Regional Lymph Nodes (N) | |
| Clinical | |
| N X | Regional lymph nodes cannot be assessed (e.g., previously removed) |
| N0 | No regional lymph node metastasis |
| N1 | Metastases to movable ipsilateral level I, II axillary lymph node(s) |
| N2 | Metastases in ipsilateral level I, II axillary lymph node(s) that are clinically fixed or matted, or in clinically detected ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases |
| N2a | Metastases in ipsilateral level I, II axillary lymph nodes fixed to one another (matted) or to other structures |
| N2b | Metastases only in clinically detected ipsilateral internal mammary nodes and in the absence of clinically evident level I, II axillary lymph node metastases |
| N3 | Metastases in ipsilateral infraclavicular (level III axillary) lymph node(s) with or without level I, II axillary lymph node involvement; or in clinically detected ipsilateral internal mammary lymph nodes and with clinically evident level I, II axillary lymph node metastases; or metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement |
| N3a | Metastases in ipsilateral infraclavicular lymph node(s) |
| N3b | Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s) |
| N3c | Metastases in ipsilateral supraclavicular lymph node(s) |
| Pathologic classification (pN) | |
| pNX | Regional lymph nodes cannot be assessed (e.g., previously removed or not removed for pathologic study) |
| pN0 | No regional lymph node metastasis identified histologically |
| pN0(i-) | No regional lymph node metastases histologically, negative IHC |
| pN0(i+) | Malignant cells in regional lymph node(s) no greater than 0.2 mm (detected by H&E or IHC including ITC) |
| pN0(mol-) | No regional lymph node metastases histologically, negative molecular findings (RT-PCR) |
| pN0(mol+) | Positive molecular findings (RT-PCR), but no regional lymph node metastases detected by histology or IHC |
| pN1 | Micrometastases; or metastases in 1 to 3 axillary lymph nodes; and/or in internal mammary nodes with metastases detected by sentinel lymph node biopsy but not clinically detected |
| pN1mi | Micrometastasis (>0.2 mm and/or more than 200 cells, but none >2.0 mm) |
| pN1a | Metastases in 1 to 3 axillary lymph nodes, at least one metastasis >2.0 mm |
| pN1b | Metastases in internal mammary nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically apparent |
| pN1c | Metastases in 1 to 3 axillary lymph nodes, and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected |
| pN2 | Metastases in 4 to 9 axillary lymph nodes; or in clinically detected internal mammary lymph nodes in the absence of axillary lymph node metastases |
| pN2a | Metastases in 4 to 9 axillary lymph nodes (at least one tumor deposit >2.0 mm) |
| pN2b | Metastases in clinically detected internal mammary lymph nodes in the absence of axillary lymph node metastases |
| pN3 | Metastases in ten or more axillary lymph nodes; or in infraclavicular (level III axillary) lymph nodes; or in clinically detected ipsilateral internal mammary lymph nodes in the presence of one or more positive level I, II axillary lymph nodes; or in more than three axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected; or in ipsilateral supraclavicular lymph nodes |
| pN3a | Metastases in ten or more axillary lymph nodes (at least one tumor deposit greater than 2.0 mm); or metastasis to the infraclavicular (level III axillary lymph) nodes |
| pN3b | Metastases in clinically detected ipsilateral internal mammary nodes in the presence of one or more positive axillary lymph nodes; or in more than three axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected |
| pN3c | Metastases in ipsilateral supraclavicular node(s) |
| Distant metastasis (M) | |
| M0 | No clinical or radiologic evidence of distant metastasis |
| cM0(i+) | No clinical or radiographic evidence of distant metastases, but deposits of molecularly or microscopically detected tumor cells in circulating blood, bone marrow, or other nonregional nodal tissue that are no larger than 0.2 mm in a patient without symptoms or signs of metastases |
| M1 | Distant detectable metastases as determined by classic clinical and radiographic means and/or histologically proven larger than 0.2 mm |
From Edge SB, Byrd DB, Compton CC, et al., eds. Breast. In AJCC cancer staging manual. 7th ed. New York: Springer, 2010:419–460, withpermission. | |
Table 40.3 Staging of Breast Carcinoma Anatomic Stage/Prognostic Groups
Treatment
Preoperative Evaluation
The extent of the preoperative workup varies with the initial stage of the disease (30). For most patients with small tumors, clinically negative lymph nodes, and no evidence of metastasis (TNM stage I), the preoperative evaluation should consist of bilateral mammography, chest radiography, complete blood count, and screening blood chemistry tests. Bone, CT, and MRI scanning are unnecessary unless there are symptoms or abnormal blood chemistry levels to suggest the existence of bone or intra-abdominal involvement. For patients with clinical stage II, node-positive disease, a bone scan is recommended, but CT scan of the abdomen is not necessary unless symptoms or laboratory results suggest liver disease. Patients with clinical stage III or stage IV disease should undergo both bone and liver scanning. PET scanning is becoming a popular means of total body scanning for breast cancer, but there have been concerns that this modality may miss some bony metastasis. A study found that PET scan is highly concordant (81%) with bone scan in a study of 132 paired studies of breast cancer patients (31). Of 31 (19%) discordant pairs, 12 patients had pathology proven metastatic disease. Nine of these 12 patients had a positive PET scan but negative bone scan, supporting the use of PET scan in detecting osseous metastasis in breast cancer, although further studies are needed to ascertain whether this modality should supplant the use of bone scan in this setting.
Figure 40.2 Appearance of breast after radical mastectomy (A) versus modified mastectomy (B). (From Kruper L, Giuliano AE. Breast disease. In: Berek JS, Hacker NF. Berek & Hacker’s Gynecologic Oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2010:636.)
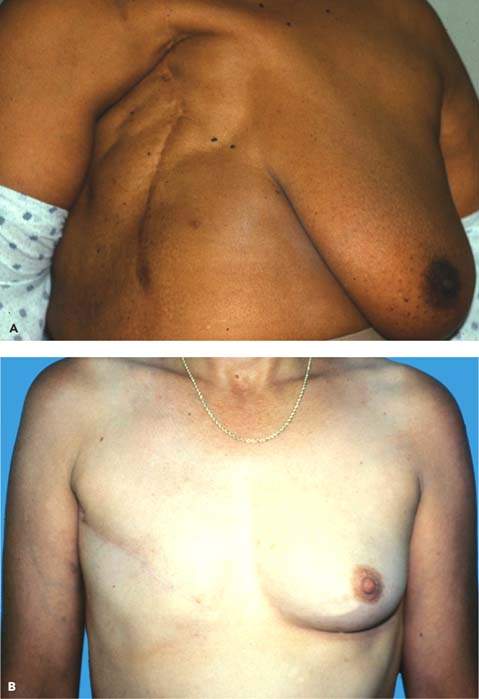
Radical Mastectomy
Traditionally, the treatment of breast cancer has been surgical, but the type of procedure has remained a controversial and highly emotional issue. During the 19th century, surgical treatment of breast cancer was haphazard, varying from local excision alone to total mastectomy. The radical mastectomy was based on the principle that breast carcinoma was a locally infiltrative process that spread in a stepwise fashion from breast, to nodes, to distant sites (32). Thus, radical mastectomy removes the entire breast, the underlying pectoral muscles, and the contiguous axillary lymph nodes in continuity (33) (Fig. 40.2A). A report of 51 years of experience with radical mastectomy, which included 1,036 patients with a follow-up of 47 years, is unequaled in evaluating any single method of treating breast cancer (34).
During the 20th century, extensions and modifications of the radical mastectomy were devised that involved removal of more local and regional tissue. At one time, supraclavicular lymph node dissections were considered a routine component of surgical treatment (35). Supraclavicular, mediastinal, and internal mammary lymph node dissections were performed (36).
An en bloc internal mammary lymph node dissection was added to the standard radical mastectomy in the 1960s (37). This technique became popular and is the operation commonly referred to as the extended radical mastectomy. Extended radical mastectomy did not enhance overall survival rates, because only 3% to 5% of patients with negative axillary nodes will have involvement of internal mammary nodes (38). Locally destructive surgery is not justified, based on current understanding of the biologic behavior of breast cancer. Radical mastectomy is no longer an indicated procedure, except in the most unusual circumstances, with extensive pectoralis involvement by direct tumor extension.
Modified Radical Mastectomy
In contrast to radical mastectomy, modified radical mastectomy preserves the pectoralis major muscle (39,40) (Fig. 40.2B). The breast is removed in a manner similar to that of radical mastectomy, but neither the axillary lymph node dissection nor the skin excision is as extensive. Consequently, there is no need for skin grafting. There are no differences in survival rates between radical and modified radical mastectomy, but the latter procedure has a better functional outcome and a superior cosmetic result (41). Modified radical mastectomy has replaced radical mastectomy in the United States and is an alternative to breast conserving surgery and axillary dissection for some patients.
Total Mastectomy
Total mastectomy involves removal of the entire breast, nipple, and areolar complex without resection of the underlying muscles or intentional excision of axillary lymph nodes. Low-lying lymph nodes in the upper outer portion of the breast and low axilla often are excised. Total mastectomy has local control rates comparable with those of radical or modified radical mastectomy but has a higher risk of axillary recurrence. In the past, regional recurrence would occur in at least 15% to 20% of patients treated with total mastectomy alone. With the addition of sentinel lymph node biopsy, which selects patients who are lymph node negative, local recurrence rates should be lowered in patients with total mastectomy and node negative disease compared to those in the past with unknown axillary status.
Skin-Sparing and Nipple-Sparing Mastectomy
More patients have early small cancers and others are undergoing prophylactic mastectomy for genetic mutations and for other high-risk lesions. Patients may elect to undergo a skin-sparing mastectomy (SSM) with nipple–areolar complex (NAC) removal that leaves a skin envelope to accommodate the breast reconstruction, as well as providing a nipple-sparing mastectomy (NSM) with preservation of the NAC. Both of these procedures are being studied for their potential utility and safety in various clinical situations. Retrospective series show that performing NSM does not impact survival, although a prospective randomized trial has not been performed (42).
Postmastectomy Radiation Therapy
McWhirter developed the combination of total mastectomy followed by radiation (43). Many advocated adjuvant radiation therapy used in combination with various operative procedures. Studies claiming improvements in overall survival usually are flawed by the use of historical controls and inaccurate preoperative staging. Classic trials, both prospective randomized and historical control studies, showed that adjuvant radiation therapy improves local control but not overall survival rates (44–47). In a prospective randomized trial performed by the National Surgical Adjuvant Breast Project (NSABP), the roles of postoperative radiation therapy and axillary treatment were examined. Patients were randomly assigned to either total mastectomy, radical mastectomy, or total mastectomy with radiation therapy. This trial showed no difference in survival among the three treatment arms, whereas radiation therapy and axillary treatment improved local and regional control. Twenty-five-year follow-up data continue to support these conclusions (48).
Three randomized control studies from the 1990s showed that postmastectomy radiation therapy reduced the risk of local–regional failure by 20% and produced an absolute survival benefit of 10% at 10 years among women with stage II to III breast cancer, regardless of menopausal status (49–51). Additional trials challenged the need for postmastectomy radiation among women with only one to three involved axillary nodes and T1 or T2 primary tumors. These studies showed adequate local–regional control rates with mastectomy and chemotherapy alone (52–54). In a large meta-analysis, an absolute risk reduction in local recurrence was found in women with radiation therapy after both breast conservation and mastectomy. One breast cancer death is avoided for every four local recurrences, reducing the 15-year mortality among patients with a greater than 10% risk of local recurrence (55). Guidelines from the American Society of Clinical Oncology recommend postmastectomy radiation therapy for women with T3 (>5 cm) primary tumors and four or more positive axillary lymph nodes (56).
Breast Conservation Therapy with or without Radiation Therapy
Radiation therapy alone, without excision of the tumor, is associated with a high local failure rate, as is local excision without radiation (57–60). Throughout the last quarter of the 20th century, a paradigm shift occurred in the surgical management of breast cancer. Data from the NSABP B-04 trial, for which 25-year follow-up exists, established the equivalency of radical versus total mastectomy with regard to overall survival. Shortly after initiation of the B-04 trial, a number of studies were designed to evaluate the efficacy of breast preservation among women with early-stage breast cancers. The Milan trial, a major prospective randomized trial that began accruing patients in 1973, compared treatment with either radical mastectomy or a combination of quadrantectomy, axillary lymph node dissection, and postoperative radiation therapy. In total, 701 clinically node-negative patients with noncentrally located, small tumors (<2 cm) (T1 N0 M0) were enrolled. After 25 years of follow-up, there continues to be no statistically significant difference between the two groups in either local control or overall survival rates (61).
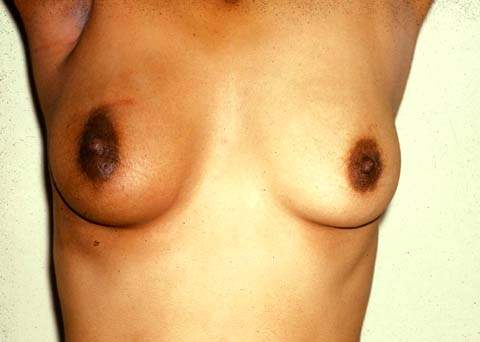
Figure 40.3 Appearance of breast after lumpectomy, axillary dissection, and radiation therapy. (From Kruper L, Giuliano AE. Breast disease. In: Berek JS, Hacker NF. Berek & Hacker’s Gynecologic Oncology. 5th ed. Philadelphia, PA: Williams & Wilkins, 2010:638.)