■ PATHOGENESIS
The absence of intracranial circulation precluding oxygen and nutrient delivery to brain tissue is the underlying common pathophysiology irrespective of the proximate cause of brain death. Hypoxia and ischemia result in brain injury and edema that continues to evolve during the intensive care unit (ICU) stay, and may ultimately progress to brain death after rostrocaudal brainstem herniation. Irreversible and total brain injury may also be present upon admission to the PICU, but can only be diagnosed as brain death after a period of observation and serial neurological examinations.
Neuropathologic findings in brain death are not diagnostic (17). Various findings described include the gross dusky, featureless pathological appearance described as “respirator brain,” brain edema, congestion, and evidence of cerebellar tonsillar herniation. These changes appear to manifest about 12 hours after ECS is observed on EEG and are unaffected by the time interval between withdrawal of ventilator support and autopsy (18). Histologically, neuronal cytoplasm may be pale and ghostlike. White matter myelin staining appears pale, and glial cell nuclei are shrunken and pyknotic. Autolysis of the cerebellar granular layer and the pituitary gland was seen in all 60 cases in one series (19). In a recent study, moderate to severe ischemic changes were observed throughout the brain. These changes occurred in the cerebral cortex and basal ganglia in 53% to 68%, thalamus in 34%, midbrain 37%, pons 37%, medulla 40%, and cerebellum in 52% of the cases (17). The lack of uniformity in brain pathology illustrates that the whole brain death definition used in the United States follows a functional rather than a structural paradigm.
■ CLINICAL COURSE AND INTENSIVE CARE UNIT MANAGEMENT
Patients who eventually progress to meet brain death criteria are often in a deep coma upon admission to the PICU or develop a comatose state while in the ICU. Close collaboration between neurologists, neurosurgeons, and pediatric intensivists is essential in providing optimal care to these critically ill infants or children. Meticulous attention to maintenance of airway, breathing, and circulation is fundamental in avoiding secondary brain injury. A detailed history of the events leading to the patient’s present condition, serial physical examination to monitor changes, and essential laboratory investigations to exclude plausible alternative diagnoses must be undertaken while stabilizing the patient in the ICU. Investigation for the cause of coma may include a blood chemistry profile with glucose, sodium, potassium, blood urea nitrogen, calcium, magnesium, and ammonia, metabolic studies, plasma and urine toxicology screens, drug levels, EEG, and neuroimaging with computed tomographic (CT) scan or magnetic resonance imaging. For a detailed approach to the initial workup and stabilization of the comatose infant or child, please refer to the Chapter “Approach to Acute Encephalopathy and Coma.”
Correction of severe electrolyte and metabolic disturbances, maintaining normothermia, assurance of absence of neuromuscular blocking agents or intoxicating substances, a normal blood pressure, and optimal oxygenation and ventilation should be undertaken by the critical care team even as potential causes of coma are being investigated. This is essential to identify and treat potentially reversible causes of coma, and also sets the stage for performing a brain death examination if the patient does not improve.
Despite optimization of physiologic parameters to maximize neuroprotection, some patients remain in or evolve to a deep unresponsive coma. These patients require a detailed neurologic examination to establish whether they meet criteria for brain death. In some cases progression to brain death may be heralded by development of a brief period of hypertension and bradycardia, signifying brainstem herniation. In other patients, some combination of pupillary changes, extreme elevation of intracranial pressure, sustained elevation of intracranial pressure above the mean arterial blood pressure precluding cerebral perfusion, development of diabetes insipidus, loss of temperature regulation, and recurrence or progression of hemodynamic instability may indicate progression to brain death. In a recent study involving adult patients, acute hypotension and polyuria signified brain death (20). Diabetes insipidus has been reported in only 38% to 41% of children with confirmed brain death and cannot be relied upon as the sole indicator of brain death (15,21). Newer modalities such as brain tissue oxygenation monitoring can also reveal the clinical progression toward brain death (22). However, the exact time-point that brain death occurs may not be clear. Indeed, the guidelines for the determination of brain death were formulated with a view to safeguard against making this diagnosis prematurely. Therefore, only after exclusion of other causes, stabilization of the patient’s hemodynamic and respiratory status, and an appropriate observation period should brain death examination and testing be undertaken.
■ DIAGNOSING BRAIN DEATH
A flow chart illustrating the process for diagnosing brain death in infants and children under the current clinical guidelines is shown in Figure 27.1 (10).
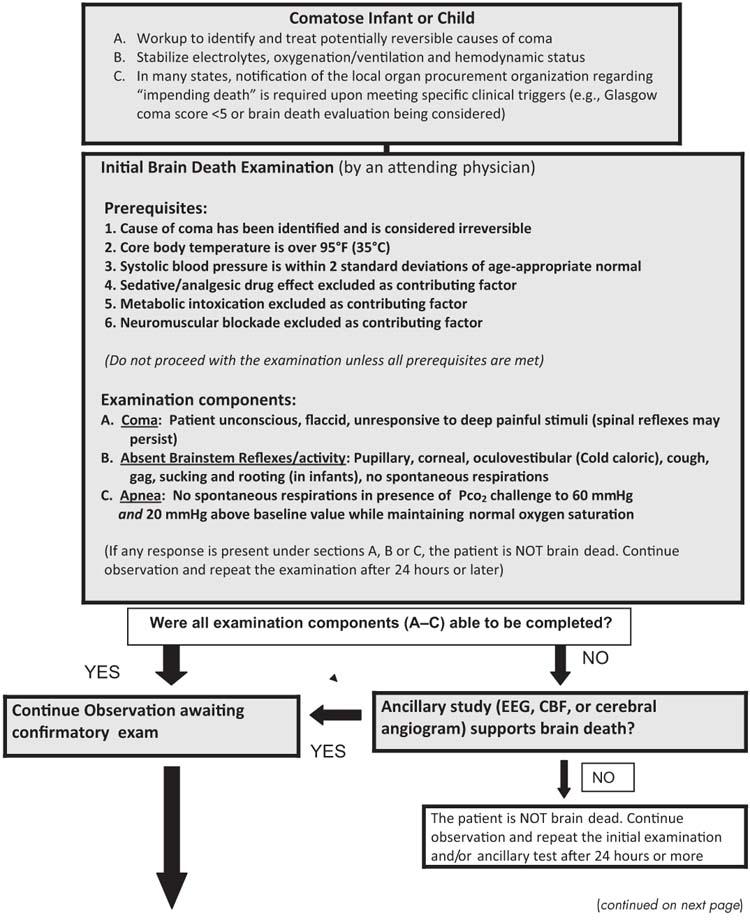
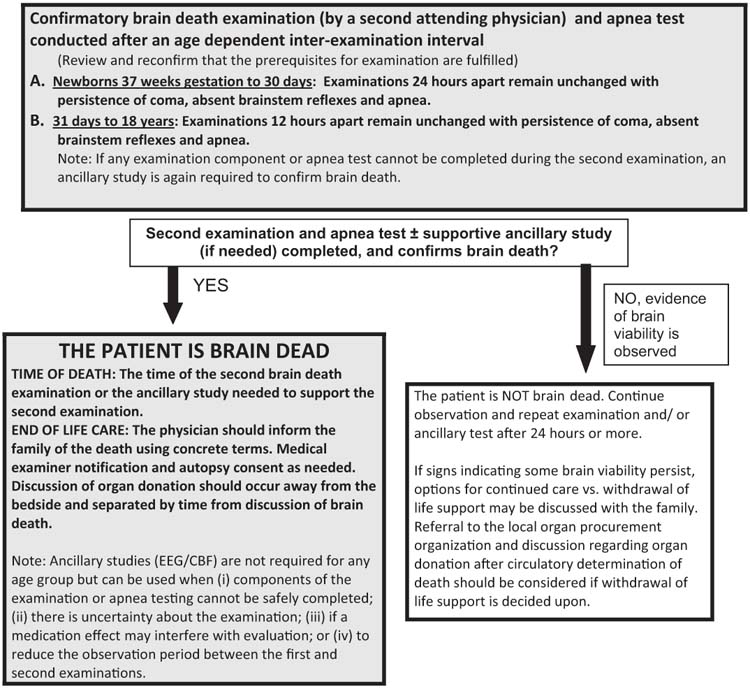
FIGURE 27.1 Evaluation for brain death in a comatose infant or child.
Based on Nakagawa TA, Ashwal S, Mathur M. et al, Guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations. Crit Care Med 2011.
Number of Examinations and Examiners
The 2011 pediatric guidelines recommend that separate attending physicians conduct the initial and confirmatory brain death examination. Apnea testing must be conducted in conjunction with each examination, but may be performed by any of the examining physicians as it is considered an objective test (10). In contrast, only one neurologic examination and apnea test is recommended according to the 2010 American Academy of Neurology guidelines to pronounce brain death in adults (6). Most brain death statutes do not specify the qualifications of the physicians performing the examination, and local hospital policies are variable (23,24). At a minimum, the physician performing brain death examination and apnea testing should have experience in performing all the components of the neurologic examination and apnea testing. The physician should also be familiar with state law and local hospital policy and procedures before conducting this examination. There is a general consensus, and in many states specific legislation (California, Louisiana, Alabama, New Jersey) that transplant surgeons not be involved in the clinical diagnosis of brain death due to an inherent conflict of interest. It is also advisable that there be agreement among the physicians caring for a particular child in the ICU setting, that the child meets criteria for brain death.
Prerequisites
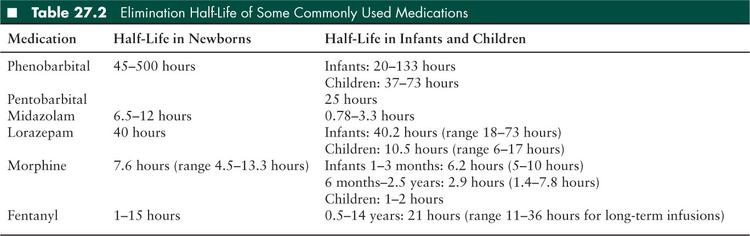
Some key prerequisites must be met before conducting brain death examination and testing in a comatose infant or child. The etiology of coma must be known, its irreversibility established, and any variables that may confound the physical examination corrected. This may require a period of observation of 24 hours or more after some inciting events such as cardiopulmonary arrest or acute traumatic brain injury in children (10). Before initiating the brain death examination, variables that can influence the clinical examination by generating an appearance of coma must be corrected to a physiologic range. These include achieving normal body temperature (using external warming if necessary) and a blood pressure that is in the normal range for age (using fluid resuscitation and/or inotropic and vasopressor support as needed). Severe electrolyte and acid-base disturbance should be corrected to physiologic values. Drug or metabolic intoxication should be excluded. Anticonvulsants, sedative medications, and neuromuscular blocking agents should be discontinued for a time period based on the half-life of the medication to ensure clearance. Elimination half-life for some medications commonly used in critical care units is provided in Table 27.2. Waiting for several half-lives in order to achieve adequate clearance of medications that may confound the clinical examination is recommended. Clinicians should keep in mind that clearance of drugs and their metabolites may be affected by factors such as the age of the child, organ dysfunction or failure, and spontaneous or induced hypothermia. Serum levels of medications with sedative properties such as barbiturates should be checked to ensure clearance to a low therapeutic range before conducting the brain death examination. Clearance of neuromuscular blocking agents can be established at the bedside by using a nerve stimulator to confirm a nondecremental “train of four” muscle twitch response (10).
Physical Examination Components
A detailed neurologic examination must be conducted to document the persistence of coma, absence of brainstem reflexes, and apnea in order to establish the diagnosis of brain death. The patients should have a flaccid tone and no spontaneous or induced movements. Response to painful stimuli, pupillary light reflex, cough, gag, corneal, oculovestibular, sucking, and rooting reflexes must all be tested systematically (10). The parts of the brain and brainstem evaluated by each component of the neurologic examination are listed in Table 27.3 and the Loma Linda Brain Death Examination form is provided in Figure 27.2. According to the current guidelines, testing the oculocephalic response is no longer required (10). The examination must remain consistent throughout the period of observation and testing. It should be noted that spinal reflexes or myoclonus may occur despite brain death, although these movements are brief, slow, and rarely persistent.
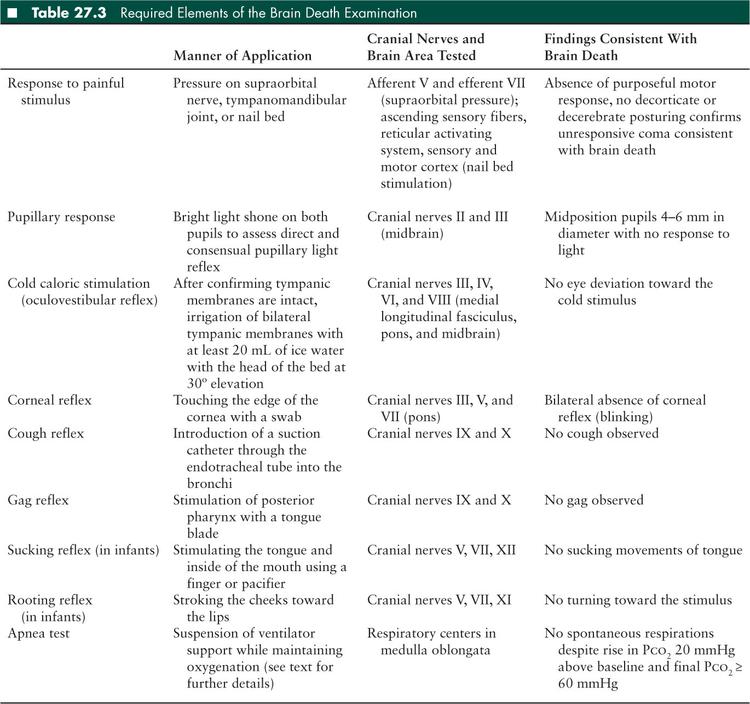
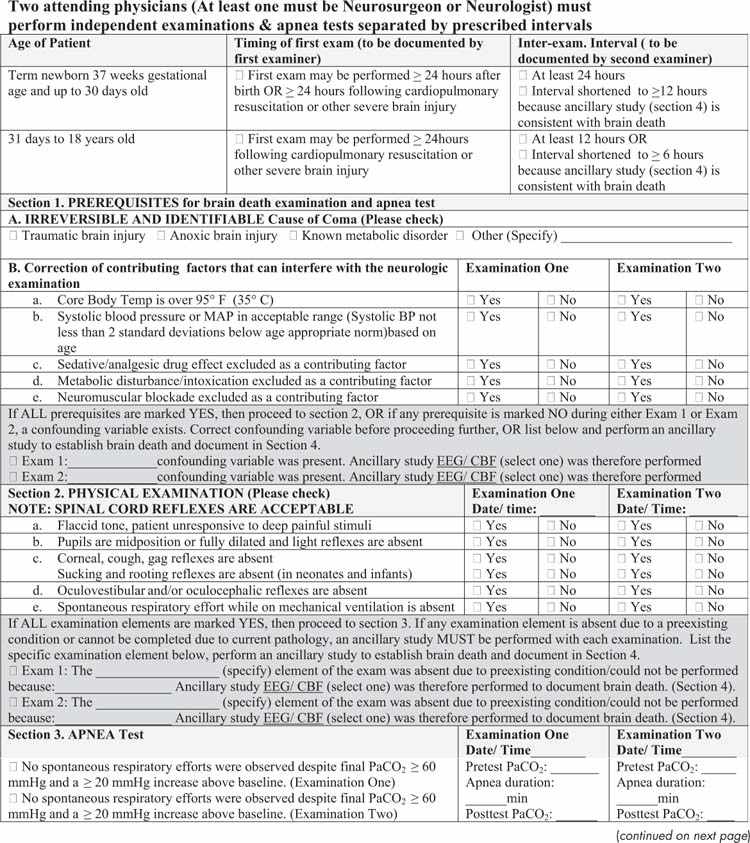
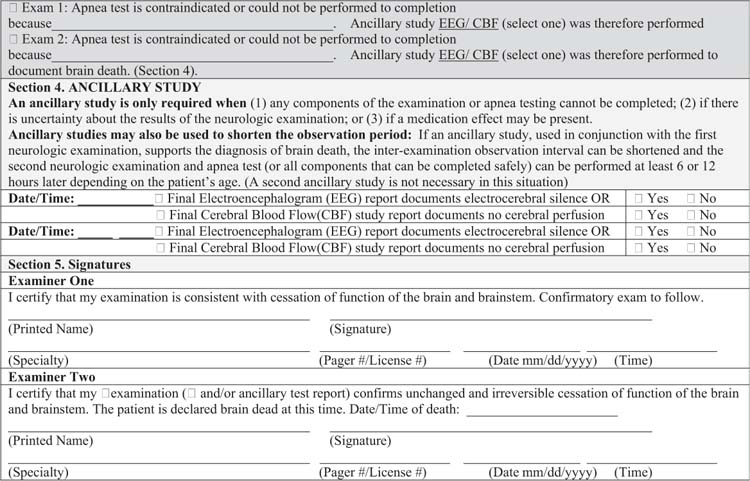
FIGURE 27.2 Brain death examination for infants and children checklist.
Apnea Testing
Current brain death guidelines specify that arterial Paco2 should rise at least 20 mmHg above the baseline and the final value should be ≥ 60 mmHg with no respiratory effort during the testing period to establish apnea consistent with brain death (10).
There are several considerations that clinicians must keep in mind in preparation for conducting an apnea test. Prior to initiating apnea testing, it is important that the critical care team assess the extent of preexisting lung injury, the ventilator settings, and hemodynamics and decide whether the patient will tolerate a prolonged period of apnea. Apnea testing should be deferred if there are conditions that would invalidate the apnea test (such as high cervical spine injury) or raise safety concerns for the patient (e.g., high oxygen requirement, high ventilator settings, high frequency oscillatory ventilation, potential for cardiovascular instability due to the respiratory acidosis inherent in apnea testing). If apnea testing is contraindicated or cannot be completed safely, an ancillary test (EEG or cerebral blood flow determination) may be used to assist with the determination of brain death. In general, the apnea test should be conducted by a physician who can not only make the assessment of pretest stability, but is also prepared to intervene if the patient has a clinical deterioration during the test. In a retrospective single center review of 228 adults with brain death, an apnea test could not be performed in 7% due to poor baseline hemodynamics or oxygenation and had to be aborted in 3% of patients due to hypoxemia or hypotension (25). In another case series, hypotension occurred in 24% and arrhythmias in 2.7% of 145 apnea tests (26). Greater alveolar-arterial oxygen gradient and acidosis preceding apnea test initiation have been associated with test completion failure in adult patients (27).
To create the safest possible environment for conducting an apnea test, the patient should be preoxygenated well. The simplest way to achieve this is to raise the Fio2 on the ventilator to 1.0 for several minutes. A baseline blood gas should be obtained and recorded. Previous studies and mathematical modeling suggest that Pco2 rises about 4 mmHg for every minute of apnea during the first 5 minutes, and 2 to 3 mmHg per minute thereafter (assuming that the patient is at a basal metabolic rate). Thus, reaching the Pco2 threshold of 60 mmHg necessary to corroborate brain death requires at least 5 minutes of apnea in initially normocapnic infants and children (28–30). To minimize the period of apnea, a baseline blood gas should be obtained, and ventilator settings adjusted so the baseline Pco2 is as close to 40 mmHg as possible. Monitoring transcutaneous Pco2 during apnea testing may help determine when the threshold of 60 mmHg has been reached, thereby limiting the occurrence of complications related to prolonged apnea (31).
The patient’s upper body and abdominal area should be exposed completely to observe for any spontaneous respiratory effort throughout the apnea test. The patient can then be disconnected from the ventilator completely, connected to a T-piece attached to the endotracheal tube (ETT) or a self-inflating bag valve system such as a Mapleson circuit connected to the ETT; or maintained on continuous positive airway pressure (CPAP) mode. CPAP may provide better maintenance of oxygenation during apnea than using a T-piece (32). Tracheal insufflation of oxygen at 6 L/min using a catheter inserted through the ETT to provide apneic oxygenation has also been described, however, if this catheter obstructs the ETT significantly, barotrauma may result (33,34). Bedside monitor tracings or ventilator triggering should not be relied upon to identify respiratory effort as transmitted cardiac pulsations or a large air leak around the ETT can sometimes create a false impression of spontaneous breathing. Caution should also be observed if the CPAP mode is used during apnea testing as some ventilators may have default settings that provide an assisted breath following a preset period of apnea or a sensitive flow-by trigger mechanism giving the false appearance of a patient-initiated respiratory effort (35,36).
A follow-up blood gas should be obtained again after about 5 minutes of apnea. If the rise in Pco2 does not meet the threshold described and the patient is clinically stable, the apnea test should be continued and blood gases repeated every few minutes until an appropriate rise in Pco2 has occurred. The baseline and final blood gas Pco2 and the period of apnea should be recorded. Oxygen saturation should be monitored closely throughout the apnea testing. If the patient develops significant arterial desaturation or cardiovascular instability at any point, apnea testing should be stopped. The patient should be provided manually assisted ventilation immediately and placed back on the ventilator. In such cases where apnea testing cannot be completed safely, ancillary testing may be pursued to assist with diagnosing brain death (10).
Interexamination Interval, Second Brain Death Examination, and Time of Death
The current guidelines recommend that a second brain death examination and apnea test be conducted to confirm brain death after a 24 hour interval in term newborns (> 37 weeks gestation) up to the age of 30 days (recommendations for preterm neonates < 36 weeks gestation were not made because of insufficient data). Twelve hours of observation are indicated for patients 31 days or older up to the age of 18 years (10). Longer intervals are recommended for younger infants, reflecting a more cautious approach to the diagnosis of brain death in this population. The recommended intervals could be shortened if an ancillary test is conducted. The physician performing the second examination should again carefully review the patient’s clinical records to ensure that there have not been any interval changes in the neurologic status. Prerequisites for brain death examination and testing as outlined previously must be reconfirmed. All elements of the neurologic examination and apnea testing should be repeated. The continued absence of all cortical and brainstem function confirms brain death. An ancillary study should be pursued in situations where a specific part of the clinical assessment cannot be performed or is unreliable. The time of the second examination confirming brain death (or of the supportive ancillary study if needed) is the legal time of death (10).
Ancillary Studies
Ancillary tests are not required for determination of brain death in infants and children under the 2011 guidelines. An ancillary study is required only if (1) any components of the examination or apnea testing cannot be completed; (2) there is uncertainty about the results of the neurologic examination; or (3) a medication effect may be present. Ancillary studies may also be used to shorten the interexamination interval to accelerate the determination of death. If the first neurologic examination and an ancillary study both support the diagnosis of brain death, the second neurologic examination and apnea test (or components that can be completed safely) can be performed at any time thereafter (10).
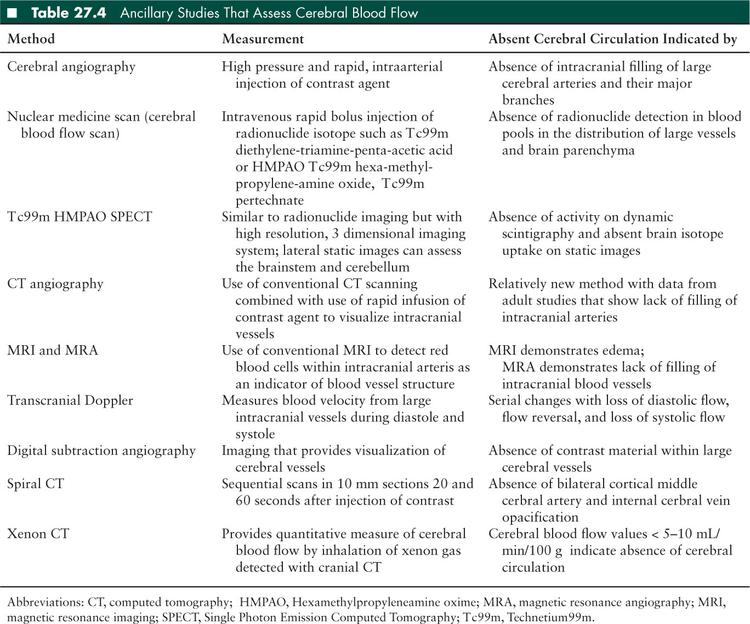
Ancillary studies used to support a diagnosis of brain death can be divided broadly into tests assessing cerebral blood flow (Table 27.4) and those assessing brain activity: EEG, brainstem auditory evoked potentials (BAERs), and somatosensory evoked potentials (SSEPs) (6,10). The choice of the test performed depends on clinician preference, institutional availability, as well as the advantages and disadvantages associated with each ancillary test in the context of a specific patient. Some of the commonly used tests are discussed further.
Electroencephalography
There are several advantages of using EEG as an ancillary test for brain death determination. It is portable, noninvasive, available at most hospitals, and relatively inexpensive. However, there are several problems which complicate the use of EEG. First, EEG tracings can be affected by electrical interference from bedside monitors and equipment at the highly sensitive thresholds set for brain death recordings (37,38). Second, an isoelectric EEG may occur soon after a cardiac arrest if sedative or anesthetic agents (e.g., barbiturates, benzodiazepines, narcotics, thiopental, ketamine, halothane, and isoflurane) are still impacting the brain (39). The American Electroencephalographic Society Guidelines have recommended criteria for brain death recordings (40). The EEG recording should be isoelectric for a minimum of 30 minutes and show no electrical activity beyond 2 μV at a sensitivity of 2 μV/mm, with filter settings at 0.1 or 0.3 second at 70 Hz.
Measuring Cerebral Blood Flow: Angiography and Radionuclide Cerebral Imaging
The technique of assessing cerebral blood flow (CBF)using radionuclide cerebral imaging has been evaluated against 4-vessel dye contrast arteriography (cerebral angiogram) in children and shows complete agreement (41,42). Although cerebral angiogram has been considered the gold standard for ancillary testing as it directly evaluates the intracranial circulation, it is rarely performed in clinical practice (10). It is expensive, invasive, time consuming, and requires the patient to be moved to the angiography suite. There are risks associated with contrast material itself such as allergic reaction or potential renal damage. These drawbacks have led clinicians to increasingly use CBF as the ancillary test for direct evaluation of cerebral blood flow. The advantages of CBF include that it is noninvasive, portable to the patient’s bedside, and has lower costs. CBF has an important added benefit similar to cerebral angiography in that it is unaffected by the presence of sedative medications that obscure the clinical and EEG examination (43–45).
EEG vs CBF
Although EEG continues to be a reliable modality, contemporary practice appears to be shifting toward the use of CBF as the preferred ancillary test in infants and children (46,47). It should be noted that though CBF may be more commonly utilized, the diagnostic yield when both EEG and CBF are initially performed is about 70% for either study in infants and children older than 1 month of age (10). For newborns, CBF should be the preferred modality as EEG with ECS is less sensitive (40%) than absence of CBF (63%) when confirming the diagnosis of brain death (10).
Other Ancillary Tests
Bispectral (BIS) monitoring: BIS is an EEG-derived method of assessing brain activity and may be useful in assessing progression to brain death (48,49). However, being EEG derived, it shares all its disadvantages. Also, BIS readings may decrease to zero prior to brain death (50). Thus, its utility as a definitive ancillary test appears to be limited at this time.
Brainstem auditory and somatosensory evoked potentials: The role of BAERs and SSEPs in brain death determination have been studied in children (51,52) but are not among the recommended ancillary tests in the current guidelines (10).
CT angiography: CT angiogram is a rapid, less invasive test with wider availability than conventional angiography, though it still requires patient transport. It has been compared directly against a nuclear medicine perfusion scan in adult subjects for brain death confirmation. CT angiogram appeared to be an efficient test with no demonstrated false negatives; and an increased sensitivity to detecting minimal cerebral blood flow in patients with open skull defects (53). In another analysis, its sensitivity was 85.7% and specificity 100% in confirming brain death (54). However, some other studies have described poor sensitivity and concordance with 4-vessel cerebral angiography (55,56).
Transcranial Doppler: The observation of reverberant flow and short systolic spikes on transcranial Doppler are indicative of absence of cerebral blood flow. This technique is easier to perform in pediatric and neonatal patients due to thin temporal bone windows; however, the presence of nonossified fontanelles in infants may alter the observed flow pattern and affect interpretation (57,58).
Brain tissue oxygenation: This technique uses invasive monitors that monitor brain tissue oxygenation directly. These monitors have been used to optimize neurocritical care therapy in patients with elevated intracranial pressure. Incidentally, it was observed that brain tissue oxygenation decreased to zero in five children who progressed to brain death, and remained above this threshold in 80 others who did not (59). Comparative data against established modalities is needed to evaluate the utility of this ancillary test.
■ END-OF-LIFE CONSIDERATIONS
Communication With the Family
The role of the critical care and neuroscience physicians is not complete after the confirmatory brain death examination. Lay persons have a poor understanding of brain death, and families may question the diagnosis of death. Their child continues to feel warm to touch, has a heartbeat, and is breathing though all these functions are maintained only as a result of technological ICU support. In one study, only half the families surveyed reported receiving an explanation regarding brain death and in others only one-third of families understood that death had occurred (60–62). It is important for the physician to use concrete terms in conveying the diagnosis of death to the family. Allowing the family to be present during the examination and apnea testing, allowing time for them to understand the diagnosis, and enlisting the assistance of organ procurement organization family care support personnel may help in this process.
In some situations, the critical care team may have to balance the added time needed for the family to come to terms with the diagnosis of brain death against scarce ICU resources needed to assist other critically ill children. For example, California law requires hospitals to consider the needs of other patients and prospective patients in urgent need of care while providing a “reasonably brief” period of accommodation for family or next of kin to gather at the patient’s bedside. No new medical intervention is required during this period, though previous cardiopulmonary support is continued (63).
Consideration of Organ Donation
The family must be given time to understand that their loved one has died before any discussions regarding organ donation. “Uncoupling” the request for donation from the pronouncement of death and a trained requestor making the request in a private setting improve consent rates for donation (64). Early referral to the designated organ procurement organization and the presence of an in-house organ procurement organization representative to coordinate the request for donation may be helpful in improving donation rates (65, 66). Approval from the coroner/medical examiner’s office should be obtained in cases of suspected child abuse before organ donation can proceed. If the family declines organ donation, ventilator support can be discontinued at any time thereafter.
Organ Donor Management
Hypotension, hypothermia, diabetes insipidus, hypernatremia, hyperglycemia, pulmonary edema, pneumonia, and coagulopathy may all occur in brain dead children and require ongoing management when organ donation is being considered (67). Such management may include the initiation of hormonal replacement therapy with thyroxine, vasopressin, and corticosteroids as well as precise hemodynamic titration and ventilator adjustments that optimize organ function (68–71). A detailed discussion of organ donor management is beyond the scope of this chapter. Ideally, the critical care team should continue to provide consultation to the personnel managing the potential donor. Aggressive and protocol-driven donor management has been shown to improve donor stability and increase organ yield (72–74). Ultimately, optimal donor management has the potential to help the thousands of potential recipients waitlisted for solid organ transplantation in the United States.
Patients Who Do Not Fulfill Brain Death Criteria
Some children who undergo brain death examination may have remnants of brain function, with clinical examination findings or ancillary test results that do not support the diagnosis of brain death. The clinicians must discuss these findings and probable long-term prognosis with the family. Magnetic resonance spectroscopy may provide additional information and assist with prognostication (75–76). Options for long-term placement in a chronic care facility after surgical placement of tracheostomy and feeding tubes versus withdrawal of life support should be discussed. If the family elects to withdraw life support, the local organ procurement organization should be consulted again as organ donation after circulatory determination of death may be a consideration.
■ SPECIAL SITUATIONS AND FUTURE DIRECTIONS
Lazarus Sign
Unusual spontaneous movements described in brain dead patients typically occurring during terminal apnea after disconnection from the ventilator are sometimes referred to as the “Lazarus sign.” These may include finger jerks, triple flexion response, arching of the back, neck turning, stiffening of the legs, and upper extremity flexion. These spinal movements can be categorized into monosegmental muscle stretch reflexes, oligosegmental cutaneomuscular reflexes, and polysegmental spinal automatism patterns (77). They may produce distress among health care personnel or family members, but these nonpurposeful stereotypical movements are spinal cord induced and in no way inconsistent with the diagnosis of brain death (78–81).
Pitfalls in the Diagnosis of Brain Death
Cases of drug intoxication, delayed clearance of medications (such as barbiturates, tricyclic antidepressants, baclofen, lidocaine, neuromuscular blockers), severe hypothermia, locked-in syndrome, Guillain-Barré syndrome or other acute neuromuscular disorders involving central and peripheral nerves, brainstem encephalitis, organophosphate poisoning, and high spinal cord injury can all appear clinically similar to brain death (82–88). In most of the reported cases of apparent “brain death,” at least one discrepancy from established brain death examination findings can be identified. Appropriate laboratory tests such as plasma drug screening to characterize the unknown agent and neuroimaging including the cervical spinal cord should be conducted if there are inconsistencies in the patient’s clinical history or neurologic examination. It is imperative that the patient be observed for a longer period, the clinical examination repeated, and ancillary studies obtained if the proximate cause of coma is unclear. The advent of new treatment modalities such as therapeutic hypothermia after cardiac arrest may also change the severity of brain injury, or the time course in which it manifests (89–90). Clinicians should extend the observation period as necessary, or obtain an ancillary test to diagnose brain death under such circumstances (10). By carefully following these principles, an inaccurate diagnosis of brain death can be avoided.
Improving Consistency in the Implementation of Brain Death Guidelines
Physician documentation of clinical examination and ancillary tests used for determining brain death is inconsistent, and frequently incomplete in both adults and children (47,91). The Presidential Commission’s goals were to develop guidelines that are clear, consistent, uniform, and reliable for diagnosis, declaration, documentation, and reporting of brain death. Previous pediatric guidelines were also intended to fit these needs, but in practice have not proven to do so as individual providers variably translated them into clinical practice (46,47,92). Codifying brainstem death guidelines into the simpler format of a checklist has proved useful in improving documentation of this crucial determination in the United Kingdom in two of three reported studies (93–95). A checklist is also provided in the Canadian forum’s recommendations for neurologic determination of death, although there are no published data describing its utilization and effectiveness (96). There is emerging data suggesting that a computerized template or checklist may be useful in improving the consistency of brain death documentation in infants and children at the hospital level in the United States (97,98). Using the checklist included with the 2011 update of the pediatric brain death guidelines could standardize their implementation across variable state laws, hospital policies, and individual preferences, making the documentation of this crucial determination more uniform nationally. A sample checklist based on the 2011 guidelines is shown in Figure 27.2. Accuracy in the determination and documentation of brain death is important to retain the confidence of our communities in the medical profession and the process of organ donation and transplantation.
■ CONCLUSIONS
Brain death is a clinical diagnosis made in a patient with coma and apnea after a period of observation and systematic evaluation and exclusion of confounding factors. The clinical examination must demonstrate complete cessation of functions of the entire brain including the brainstem. Two complete neurologic examinations and apnea tests separated by age-dependent intervals must be performed to establish brain death. Using a checklist based on the current brain death guidelines may help to standardize their implementation and documentation across variable state laws, hospital policies, and individual preferences. Neurocritical care specialists must provide a supportive environment while communicating the diagnosis of brain death to the family using unambiguous terms. End of life options such as autopsy and organ donation should be discussed at a later time once the family has understood that their child has died. Further care decisions regarding patients who do not fulfill brain death criteria should be made considering the family’s wishes, and may include withdrawal of life support or ongoing chronic care in a specialized long-term care facility.
■ REFERENCES
1. , . Le coma depasse. Rev Neurol. 1959;101:3–15.
2. A definition of irreversible coma; report of Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA. 1968;205:337–340.
3. Collaborative study on brain death: an appraisal of the criteria of cerebral death: A summary statement. JAMA. 1977;237:982–986.
4. Guidelines for the determination of death. Report of the Medical Consultants on the Diagnosis of Death to the President’s Commission for the Study of Ethical Problems in Biomedical and Behavioral Research. JAMA. 1981;246(19):2184–2186.
5. The Quality Standards Subcommittee of the American Academy of Neurology. Practice parameters for determining brain death in adults (summary statement). Neurology. 1995;45:1012–1014.
6. , , . Determining brain death in adults: 2009 guideline update. Neurology. 2010;74:1911–1918.
7. Report of Special Task Force: Guidelines for determination of brain death in children. Pediatrics. 1987;80:298–300.
8. Guidelines for determination of brain death in children. Pediatr Neurol. 1987;3(4):242–243.
9. , . Brain death in the newborn: clinical, EEG and blood flow determinations. Pediatrics. 1989;84:429.
10. et al. Guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations. Crit Care Med. 2011;39:2139–2155.
11. Uniform Brain Death Act. Uniform Laws annot 12:63 (west 1993; west suppl. 1997). Paper presented at: National Conference of Commissioners of Uniform State Laws Meeting; 1980; Kauai, HI; p2.
12. Determination of death. 10 N.Y.C.R.R. § 400.16, e(3).
13. New Jersey Declaration of Death Act. Death not declared in violation of individual’s religious beliefs. L1991. ch 90; NJSA 26:6A–5.
14. President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Defining Death: Medical, Legal and Ethical Issues in the Determination of Death. Washington, DC: US Government Printing Office, 1981.
15. et al. Brain death in pediatric intensive care unit patients: incidence, primary diagnosis, and the clinical occurrence of Turner’s triad. Crit Care Med. 1994;22(8):1301–1305.
16. et al. Modes of death in the pediatric intensive care unit: withdrawal and limitation of supportive care. Crit Care Med. 1993;21:1798–1802.
17. , . Neuropathology of brain death in the modern transplant era. Neurology. 2008;70:1234–1237.
18. , , . Temporal correlates in brain death. EEG and Clinical relationships to the respirator brain. Arch Neurol. 1984;41:147–152.
19. , , . A clinico-neuropathological study on brain death. Nagoya J Med Sci. 1993;56(1-4):89–99.
20. et al. Pronouncing brain death. Contemporary practice and safety of the apnea test. Neurology. 2008;71:1240–1244.
21. et al. Diabetes insipidus in children with brain death. Crit Care Med. 1987;15(6):551–553.
22. , . Brain tissue oxygenation in brain death. Neurocrit Care. 2005;2:17–22.
23. et al. Variability of brain death determination guidelines in leading neurologic institutions. Neurology. 2008;70:284–289.
24. , , . Variability among hospital policies for determining brain death in adults. Crit Care Med. 2004;32:1284–1288.
25. et al. Pronouncing brain death. Contemporary practice and safety of the apnea test. Neurology. 2008;71:240–1244.
26. , , . Complications during apnea testing in brain death-predisposing factors. Neurology. 2000;55(7)1045–1048.
27. et al. Predictors of apnea test failure during brain death determination. Neurocrit Care. 2010;12:352–355.
28. , . Apnea documentation for determination of brain death in children. Pediatrics. 1984;74:505–508.
29. , . Apnea testing in suspected brain dead children—physiological and mathematical modeling. Intensive Care Med. 1995;21:247–252.
30. , . Apnea testing to confirm brain death in children. Crit Care Med. 1984;12:357–358.
31. et al. An evaluation of transcutaneous carbon dioxide partial pressure monitoring during apnea testing in brain-dead patients. Anesthesiology. 2006;104(4):701–707.
32. et al. Efficacy of a T-piece system and a continuous positive airway pressure system for apnea testing in the diagnosis of brain death. Crit Care Med. 2006;34(8):2213–2216.
33. , , . Tension pneumothorax during apnea testing for the determination of brain death. Anesthesiology. 1998;89:1250–1251.
34. , . Barotrauma during apnoea testing for brain death determination in a five-year-old boy. Anaesth Intens Care. 2008;36(3):462–463.
35. , , . Ventilator self-cycling may falsely suggest patient effort during brain death determination. Neurology. 2005;65(5):774.
36. , . Brain death and ventilator trigger settings. Anaesthesia. 2000;55:676–684.
37. . Usefulness of EEG in the evaluation of brain death in children: the pros. Electroencephalogr Clin Neurophysiol. 1989;73:272–274.
38. . Usefulness of EEG in the evaluation of brain death in children: the cons. Electroencephalogr Clin Neurophysiol. 1989;73:276–278.
39. et al. Resuscitation after severe hypoxia in a young child: temporary isoelectric EEG and loss of BAEP components. Intensive Care Med. 1993;19:420–422.
40. American Electroencephalographic Society. Guideline three: minimum technical standards for EEG recording in suspected cerebral death. J Clin Neurophysiol. 2006;23(2):97–104.
41. Schwartz JA, Baxter J, Brill DA. Diagnosis of brain death in children by radionuclide cerebral imaging. Pediatrics. 1984;73(1):14–18.
42. et al. Usefulness of (Tc99m) HM-PAO scan in supporting clinical brain death in children: uncoupling flow and function. Clin Intensive Care. 1994;5(2):71–74.
43. , . Scintigraphy as a confirmatory test of brain death. Semin Nucl Med. 2003;33(4):312–323.
44. , , . Confirmation of brain death with portable isotope angiography: a review of 204 consecutive cases. Neurosurgery. 1985;16(4):492–497.
45. et al. Radionuclide cerebral perfusion scintigraphy in determination of brain death in children. Neurology. 1983;33(8):1027–1031.
46. , . Variability in brain death determination practices in children. JAMA. 1995;274(7):550–553.
47. et al. Variability in pediatric brain death determination and documentation in southern California. Pediatrics. 2008;121(5):988–993.
48. et al. Bispectral EEG monitoring for early detection of brain death. Transpl Proc. 2008;40(5):1279–1281.
49. et al. Bispectral index monitoring in confirmation of brain death in children. J Child Neurol. 2006;21:799–801.
50. et al. Detection of brain death onset using the bispectral index in severely comatose patients. Intensive Care Med. 2002;28:419–425.
51. et al. Brain death in children: clinical, neurophysiological and radioisotopic angiography findings in 125 patients. Childs Nerv Syst. 2000;16:40–45.
52. et al. Brain death and evoked potentials in pediatric patients. Crit Care Med. 1999;27:412–416.
53. , , . Brain death confirmation: comparison of computed tomographic angiography with nuclear medicine perfusion scan. J Trauma. 2010;68:553–559.
54. et al. CT angiography for brain death diagnosis. Am J Neuroradiol. 2009;30:1566–1570.
55. et al. Limitations of computerized tomographic angiography in the diagnosis of brain death. Intensive Care Med. 2007;33:2129–2135.
56. et al. Reliability of computed tomographic angiography in the diagnosis of brain death. Transpl Proc. 2007;39:16–20.
57. , . Sensitivity of transcranial Doppler for confirming brain death: a prospective study of 270 cases. Acta Neurol Scand. 2006;113:426–432.
58. et al. Transcranial Doppler for brain death in infants: the role of the fontanelles. Eur Neurol. 2010;63:164–169.
59. , . Brain tissue oxygenation in children diagnosed with brain death. Neurocrit Care. 2010;12:56–61.
60. et al. Explaining brain death: a critical feature of the donation process. J Transpl Coord. 1997;7(1):14–21.
61. et al. A survey of families of brain dead patients: their experiences, attitudes to organ donation and transplantation. Anaesth Intensive Care. 1995;23(1):88–95.
62. , , . Families’ understanding of brain death. Prog Transplant. 2003;13(3):218–224.
63. CA health and safety code, section 1254.4.
64. et al. Improving the request process to increase family consent for organ donation. J Transpl Coord. 1998;8(4):210–217.
65. et al. Factors influencing families’ consent for donation of solid organs for transplantation. JAMA. 2001;286(1):71–77.
66. et al. Location of in-house organ procurement organization staff in level I trauma centers increases conversion of potential donors to actual donors. Transplantation. 2003;75:1330–1335.
67. , , . Care of the potential pediatric organ donor. Pediatr Clin North Am. 2001;48:715–749.
68. et al. Vasopressin pressor effects in critically ill children during evaluation for brain death and organ recovery. Resuscitation. 2000;47:33–40.
69. et al. The effect of a thyroid hormone infusion on vasopressor support in critically ill children with cessation of neurologic function. Crit Care Med. 2004;32:2318–2322.
70. et al. Effects of catecholamine application to brain-dead donors on graft survival in solid organ transplantation. Transplantation. 2001;72(3):455–463.
71. . Updated pediatric donor management and dosing guidelines. NATCO, the Organization for Transplant Professionals Web site. www.natco1.org/prof_development/index.htm#guidelines. Updated 2008. Accessed Nov 1, 2010.
72. et al. Increased transplanted organs from the use of a standardized donor management protocol. Am J Transplant. 2002;2:761–768.
73. et al. Intensive care management of paediatric organ donors and its effect on post-transplant organ function. Intensive Care Med. 1996;22:1424.
74. et al. Aggressive pharmacologic donor management results in more transplanted organs. Transplantation. 2003;75:482–487.
75. et al. MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J Magn Reson Imaging. 2006;24(4):801–811.
76. , , . Proton MRS in acute traumatic brain injury: role for glutamate/glutamine and choline for outcome prediction. J Neurotrauma. 2004;21(12):1693–1705.
77. et al. Phenomenological diversity of spinal reflexes in brain death. Eur J Neurol. 2000;7:315–321.
78. et al. Decerebrate-like posturing with mechanical ventilation in brain death. Neurology. 2000;54:224–227.
79. . Unusual spontaneous movements in brain-death patients. Neurology. 1984;34:1089.
80. et al. Spontaneous and reflex movement in brain death. Neurology. 2000;54:221.
81. , , . Head turning in brain death. J Clin Anaesth. 1996;8:141–143.
82. . The diagnosis of brain death. N Engl J Med. 2001;344(16):1215–1221.
83. , , . Brain death and the cervical spinal cord: a confounding factor for the clinical examination. Spinal Cord. 2010;48:2–9.
84. et al. Simulation of brain death from fulminant de-afferentation. Can J Neurol Sci. 2003;30:397–404.
85. et al. Coma mimicking brain death following baclofen overdose. Intensive Care Med. 2001;27(5):945–947.
86. , , . Extremely prolonged vecuronium clearance in a brain death case. Anesthesiology. 2001;95:1023–1024.
87. , , . In-laws, insecticide and a mimic of brain death. Lancet. 2008;371(9612):622.
88. et al. Non-barbiturate, drug induced reversible loss of brainstem reflexes. Neurology. 1998;51(2)639–640.
89. et al. A 10 month old with reversible findings of brain death. Pediatr Neurol. 2009;41(5):378–382.
90. , , . Therapeutic hypothermia after cardiac arrest: another confounding factor in brain-death testing. Pediatr Neurol. 2010;42(4):304.
91. , , . Brain death documentation: analysis and issues. Neurosurgery. 2002;51:731–736.
92. , . Can Pediatricians define and apply the concept of brain death? Pediatrics. 1999;103(6):e82.
93. , . Diagnosing brain death: the importance of documenting clinical test results. Anesthesiology. 1999;54:81–83.
94. , . An evaluation of brainstem death documentation: the importance of full documentation. Pediatr Anesth. 2004;14:584–588.
95. , . Clinical course and determination of brainstem death in a children’s hospital. Acta Paediatr. 2004;93:47–52.
96. et al. Severe brain injury to neurological determination of death: Canadian forum recommendations. Can Med Assoc J. 2006;174:S1–S12.
97. , , . Impact of a computerized note template/checklist on documented adherence to institutional criteria for determination of neurologic death in a pediatric intensive care unit. Pediatr Crit Care Med. 2011;12(3):271–276.
98. , , . Using a checklist improves pediatric brain death documentation. Crit Care Med. 2010;12(suppl):A720.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


