10 Borderline Lesions Papillomas are characterized histopathologically as intraductal tumors composed of benign epithelial cells covering a central branching fibrovascular core. They are generally differentiated to include solitary intraductal papillomas, which are typically located in the retromamillary region, and the small, usually multiple, peripheral intraductal papillomas. Papillomas must be distinguished from pseudopapillary lesions, which are intraductal hyperplasias with a papillary architecture that occur without the presence of a central fibrous core, and from the papillary adenoma of the nipple, with its combination of papillary and tubular structures. The pseudopapillary lesions include papillomatosis and juvenile papillomatosis, the latter of which commonly occurs in adolescents and young women between 10 and 40 years of age. If the histopathological results of a percutaneous breast biopsy reveal a papilloma or papillomatosis, they are usually classified into the pathological B3 category (see Table 16.1). Because of the increased tumor transformation risk, a pathological–radiological tumor conference is held to decide whether surgical excision is required. The decision is usually dependent upon the primary lesion size on imaging, and the extent of tissue sampling. If it must be assumed that the papilloma has not been sufficiently removed, surgical excision should be recommended to attempt complete excision. Small solitary intraductal papilloma. Usually not detectable (Figs. 10.1b, 10.2b). Larger solitary intraductal papilloma (> 1 cm). Round or oval lesion with isointense signal to parenchyma (Fig. 10.3b). Peripheral intraductal papillomas. Usually not detectable (Figs. 10.4b, 10.5b). Small solitary intraductal papilloma. Often occult. Otherwise usually intermediate (Fig. 10.2c) or high signal intensity (Fig. 10.1c). Larger solitary intraductal papilloma (> 1 cm). Often occult. Otherwise round or oval lesion with intermediate or high signal intensity (Fig. 10.3c). Peripheral intraductal papillomas. Usually not detectable. Occasionally high water signal within ductal structure (Figs. 10.4c, 10.5c). Small solitary intraductal papilloma. A focus or mass with increased enhancement can be detected from a size of 4–5 mm diameter (Fig. 10.1a,d). It usually has a round or oval shape, is well-circumscribed, and typically displays a homogeneous enhancement. Occasionally, displays a rim-enhancement. TIC is uninformative (Fig. 10.1e). Larger solitary intraductal papilloma (> 1 cm). Round or oval lesion that typically displays a homogeneous enhancement. Occasionally displays rim-enhancement (Fig. 10.3a,d). TIC is uninformative (Fig. 10.3e). Peripheral intraductal papillomas. Linear (Fig. 10.5d), linearbranching (Fig. 10.4d), or regional enhancement (nonmasslike) (Fig. 10.6a,d). TIC may be uninformative (Figs. 10.4e, 10.5e) or typical for malignancy (Fig. 10.6e).
Papilloma
 Papillomas are considered to be borderline epithelial lesions with an increased lifetime risk for the development of DCIS or invasive papillary breast cancer.
Papillomas are considered to be borderline epithelial lesions with an increased lifetime risk for the development of DCIS or invasive papillary breast cancer.
 MR Mammography: Papilloma
MR Mammography: Papilloma
T1-Weighted Sequence (Precontrast)
T2-Weighted Sequence
T1-Weighted Sequence (Contrast Enhanced)
 Breast MRI does not allow a reliable differentiation between a benign papilloma and a papilloma in which malignant transformation has taken place (Figs. 10.7, 10.8, 10.9).
Breast MRI does not allow a reliable differentiation between a benign papilloma and a papilloma in which malignant transformation has taken place (Figs. 10.7, 10.8, 10.9).
Incidence: | Rare, 1%–2% of all benign tumors. |
Age peak: | 40–50 years. |
Risk of malignant transformation: | Solitary papilloma: slightly increased lifetime risk (comparable with that of ADH). Multiple peripheral papillomas: increased lifetime risk of ~10%. |
Findings | |
Clinical: | Often clinically occult. Spontaneous or provokable nipple discharge (exfoliative cytology). Rarely presents as palpable mass (e.g., large retromamillary papillomas). |
Mammography: | Usually mammographically occult. Larger tumors appear well-circumscribed, homogeneously dense, round or oval. |
Galactography: | Intraductal exclusion(s) or abrupt duct truncation(s). Indication: pathological secretion. |
Ultrasonography: | Usually occult. Rarely seen as round, retromamillary lesion. |
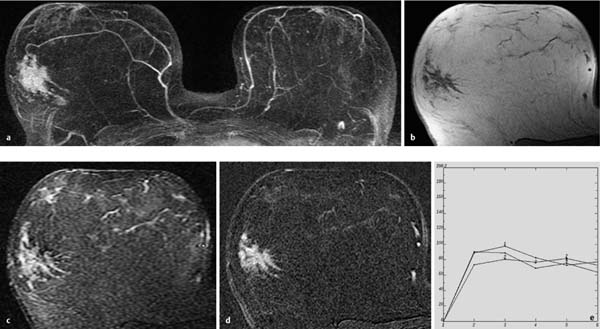
Fig. 10.1a–e Solitary peripheral papilloma.
a Subtraction MIP: solitary, small focus in the right breast.
b T1w precontrast slice image: unremarkable findings.
c IR T2w slice image: focus displays high water content.
d Early subtraction slice image: well-circumscribed, hypervascularized focus of 4 mm diameter.
e Time–signal intensity curve (TIC): uninformative.
Histology: papilloma of 4 mm diameter.
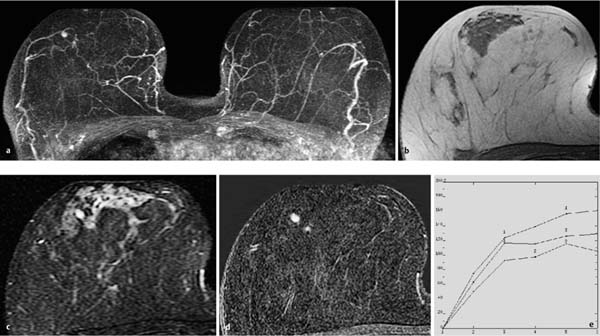
Fig. 10.2a–e Solitary retromamillary papilloma.
a Subtraction MIP: hypervascularized lesion with meandering shape in the retromamillary aspect of the left breast.
b T1w precontrast slice image: unremarkable findings.
c IR T2w slice image: intermediate signal intensity of lesion.
d Early subtraction slice image: hypervascularized, elongated, lobular finding in the retromamillary aspect of the left breast.
e TIC: uninformative.
Histology: papilloma of 7 mm diameter.
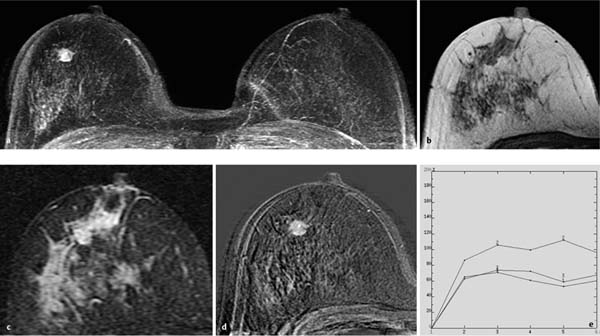
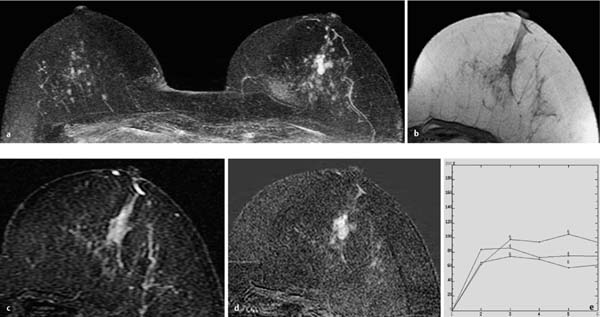
Fig. 10.3a–e Large solitary papilloma.
a Subtraction MIP: hypervascularized mass in the right breast.
b T1w precontrast slice image: unremarkable findings.
c IR T2w slice image: mass displays slightly increased signal intensity.
d Early subtraction slice image: mass of 10 mm diameter displays rim and central enhancement.
e TIC: strong initial enhancement and postinitial plateau.
Histology: papilloma of 10 mm diameter.
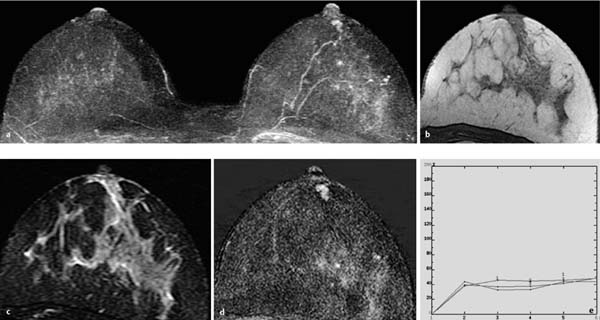
Fig. 10.4a–e Linear papillomatosis.
a Subtraction MIP: linear hypervascularization in the central aspect of the right breast. Status after mastectomy of the left breast.
b T1w precontrast slice image (right breast): unremarkable findings.
c IR T2w slice image (right breast): increased signal intensity in affected milk duct.
d Early subtraction slice image (right breast): linear-branching enhancement in the central aspect.
e TIC: uninformative.
Histology: papillomatosis.
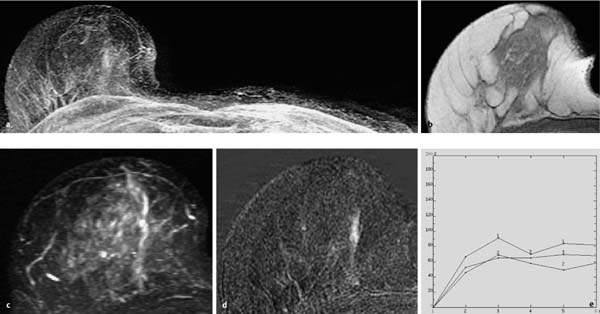
Fig. 10.5a–e Segmental peripheral papillomatosis.
a Subtraction MIP: segmental hypervascularization in the retromamillary aspect of the right breast. Multiple foci in the left breast.
b T1w precontrast slice image (right breast): discrete duct ectasia.
c IR T2w slice image (right breast): increased signal intensity in affected milk duct.
d Representative early subtraction slice image (right breast): linear enhancement along milk duct.
e TIC: uninformative.
Histology: papillomatosis.
Fig. 10.6a–e Regional peripheral papillomatosis.
a Subtraction MIP: regional hypervascularization in the medial aspect of the left breast. Unremarkable right breast.
b T1w precontrast slice image (left breast): hypointense region containing several round lesions in the medial aspect of the left breast.
c IR T2w slice image (left breast): increased signal intensity of these round lesions.
d Representative early subtraction slice image (left breast): round lesions within regional area of enhancement display no enhancement.
e TIC: suspicious curve with mild postinitial washout.
Histology: papillomatosis. No associated malignancy.
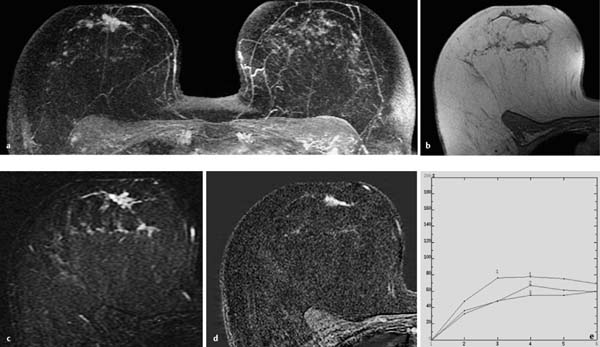
Fig. 10.7a–e Peripheral papillomatosis with ADH.
a Subtraction MIP: convolute of hypervascularized, round lesions in the central aspect of the left breast. Multiple foci in the right breast.
b T1w precontrast slice image (left breast): isointense, masslike region in the central aspect of the left breast.
c IR T2w slice image (left breast): slightly increased signal intensity in this region.
d Representative early subtraction slice image (left breast): conglomerate of hypervascularized foci and masses in the central aspect of the left breast.
e TIC: suspicious curve with mild postinitial washout.
Histology: papillomatosis with ADH.
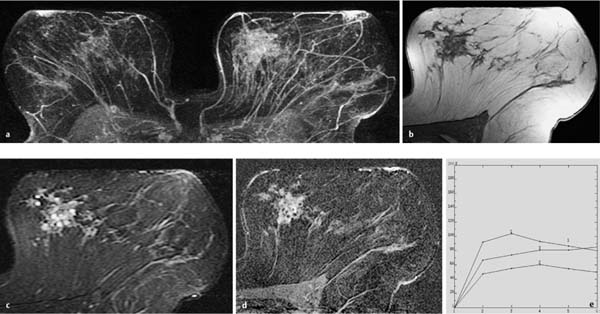
Fig. 10.8a–e Peripheral papillomatosis with ADH.
a Subtraction MIP: regional hypervascularization in the lateral aspect of the right breast. Unsuspicious hypervascularized lymph node in the left breast.
b T1w precontrast slice image (right breast): architectural distortion.
c IR T2w slice image (right breast): increased signal intensity in this region.
d Representative early subtraction slice image (right breast): spiculated, hypervascularized area in the lateral aspect of the right breast.
e TIC: suspicious curve with pronounced postinitial washout.
Histology: papillomatosis with ADH.