Fig. 7.1
Changes in total body bone mineral content (BMC) at discharge (2 months) and at 6 months post-burn, expressed as a percentage of baseline (admission) values. Data are shown as mean and standard error. Statistical significance versus control is designated by an asterisk. (Reproduced from Klein et al. [14] by permission of Springer Science + Business Media.)
Of interest is that this pattern of change was more striking in the lumbar spine, where bone mineral content at discharge increased by around 5 % in the pamidronate group (n = 17) and decreased by 7 % in the placebo group (n = 12), a difference that was statistically significant at hospital discharge. By 6 months post-burn lumbar spine bone mineral content rose by 10 % from baseline admission levels in the pamidronate group (n = 7) while stabilizing at 7 % below admission baseline in the placebo group (n = 6), also being significantly different from the pamidronate group (see Fig. 7.2).
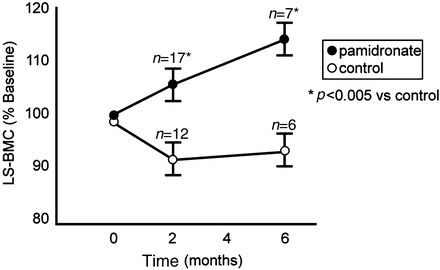

Fig. 7.2
Changes in lumbar spine (LS) bone mineral content (BMC) at discharge (2 months) and at 6 months post-burn, expressed as a percentage of baseline (admission) values. Data are shown as mean and standard error. Statistical significance versus control is designated by an asterisk. (Reproduced from Klein et al. [14] by permission of Springer Science + Business Media.)
Bone resorption at 2 weeks post-burn did not appear to differ between pamidronate and placebo groups either by measure of free deoxypyridinoline biomarkers in the urine or by quantitative iliac crest resorptive surface. This would ordinarily be surprising in light of the potential mechanisms of bone loss discussed earlier in the chapter. It is possible, however, that the evaluation methods were not ideal. In the case of the free deoxypyridinoline, it is not as sensitive to changes in resorption as total deoxypyridinoline, the assay for which was not available at the time this study was carried out. Also, the iliac crest consists of both cortical and trabecular bone, and inasmuch as cortical bone changes relatively little following burn injury compared to trabecular bone, it is possible that any increase in resorptive surface of the trabecular component may have been offset by the stability of the larger cortical component.
One other consideration regarding bone resorption must also be addressed here. That is that urine calcium excretion remained elevated in both placebo and pamidronate groups. Although the quantity of calcium excreted in the urine over 24 h was about 25 % lower in the pamidronate group at about 2 weeks post-burn the difference from the placebo group was not statistically significant. Could the increased urinary calcium excretion in both groups indicate increased resorption?
While it is tempting to think so interpretation of the hypercalciuria is confounded by the inappropriately low levels of parathyroid hormone (PTH) in the blood in response to the low circulating ionized calcium concentrations (Fig. 7.3). This hypocalcemic hypoparathyroidism, which has previously been noted [10], is likely due to the inflammatory cytokine-mediated up-regulation of the calcium-sensing receptor of the parathyroid gland, which has been documented to occur in a sheep model of burn injury [11]. The consequence of this up-regulation is that a lower, often abnormally low, level of circulating calcium is sufficient to suppress PTH secretion by the parathyroid glands and the resultant hypoparathyroidism is permissive of urinary calcium excretion. Therefore, at the present time it is unclear if the hypercalciuria reflects bone resorption or simply the oversupply of calcium given intravenously in an attempt to correct the hypocalcemia. What was shown in this present study, however, is that the amount of calcium administered to patients receiving pamidronate and those receiving placebo was not significantly different in the attempt to achieve normal circulating levels of ionized calcium. Therefore, by blocking bone resorption pamidronate is not exacerbating existing hypocalcemia and its lack of effect in reducing hypercalciuria underscores the likelihood that the source of the urinary calcium excretion is the parenterally administered calcium. One additional consideration is the timing of the urine collection. Inasmuch as patients underwent the urine collection by 10 days post-burn it is possible that at the time of collection active resorption was slowing down due to onset of osteoblast apoptosis. In this case the urinary calcium from the placebo group might have been higher if it had been quantitated earlier post-burn and the differences in urinary calcium excretion between the two groups might then have reached statistical significance.
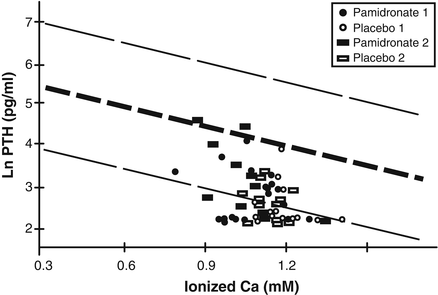

Fig. 7.3
Nomogram depicting the mean and 99 % confidence interval of the natural logarithmic (ln) PTH response to blood ionized calcium levels (expressed as mM). The majority of PTH values remain below the 99 % confidence interval for ionized calcium regardless of administration of placebo or pamidronate. The designation 1 refers to baseline values and the designation 2 refers to hospital discharge. (Reproduced from Klein et al. [14] by permission of Springer Science + Business Media.)
Now that we have addressed though not solved the bone resorption issues raised by this study, we must address the finding that pamidronate failed to prevent the loss of osteoblasts as seen on histomorphometry of the specimens taken from the iliac crest. Given this observation how do we explain the continued gain in bone mineral content over 6 months’ time in the burned children receiving pamidronate, especially in the lumbar spine region?
Studies have shown that bisphosphonates have antiapoptotic properties [16]. While this may be the case the failure to preserve osteoblasts at the bone surface at 2 weeks post-burn must be addressed. One possibility that was not examined by this study is that the antiapoptotic properties become manifest at a later time than the antiresorptive properties. This may be plausible due to initially large quantities of endogenous glucocorticoids produced by the adrenal glands in response to the stress of the burn injury [1, 4]. As these levels drop off it is possible that the antiapoptotic activity of pamidronate is unmasked. This explanation may have been better addressed by examining bone biopsies between 2 weeks and the 1 year post-burn follow-up specimens or by systematically and sequentially examining biomarkers of bone formation. These data were not available in the study discussed here. Another possible explanation would be that the antiapoptotic properties of pamidronate are more clearly discernible in purely trabecular bone, such as in lumbar spine. That possibility also needs to be pursued.
While it would appear that pamidronate acts only on bone and on no other aspect of the abnormal calcium homeostasis brought about by burn injury, data on muscle mass, which were available from total body DXA studies, were not included in the data analysis or the publication. Given the present degree of interest in the interaction between muscle and bone this oversight requires attention. While it is clear that during this 6-month time period the catabolic response and likely the heightened production of endogenous glucocorticoids as part of the stress response produce negative nitrogen balance and muscle wasting [17] it is not certain whether the antiresorptive activity of the bisphosphonates and the positive effect they have on bone mineral content by whatever mechanism could attenuate the loss of lean body mass. If not, then bisphosphonates can be directly targeted to reduce bone loss without any likelihood of affecting any other adaptive response. If there were to be an attenuation of muscle wasting concomitant with improvement in bone mineral content then perhaps bisphosphonates could play an adjunctive role in the limiting of post-burn catabolic response as well.
We will next examine the longer-term effects of the acute administration of pamidronate up to 2 years following the burn injury. Subjects were unblended after 6 months but new subjects had also been added to both groups in randomized fashion to provide a total of 57 subjects, 32 having received pamidronate and 25 having received saline placebo. Enrolled children were followed every 6 months with DXA studies. After 2 years attrition resulted in having a complete set of values in only 21 subjects, 8 of whom received pamidronate and 13 of whom received saline placebo. There appeared to be no attribution of dropout to any side effects of pamidronate.
The results demonstrated that total body bone mineral content as a percentage change from acute admission values exhibited a steady increase over the 6-month intervals until reaching 2 years post-burn in those who were given pamidronate. In contrast those subjects who had received placebo remained significantly behind in the total body accretion of bone mineral content until 18 months post-burn, at which point the percentage change from admission rapidly rose to equal the percentage change from admission in the experimental group (see Fig. 7.4).
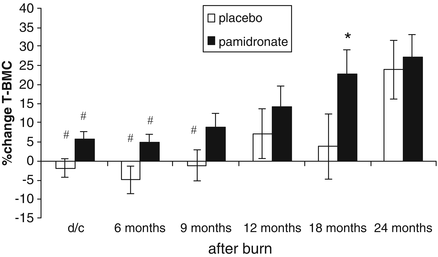

Fig. 7.4
Percent change in total bone mineral content from baseline to 24 months after burn (d/c: hospital discharge). Values are means and standard error of the means. Significant differences between placebo and pamidronate are designated by asterisk with p < 0.05. Significant time effects within a group when compared to changes at 24 months are designated by hash, with p < 0.05. (Reproduced from Przkora et al. [15] with permission of Elsevier.)
As was seen at 6 months post-burn in the acute study [14], the beneficial effect of pamidronate was more pronounced in the trabecular bone of the lumbar spine. In the lumbar spine the significant difference between pamidronate and control groups in bone mineral content but not bone area persisted for the entire 2-year follow-up period (see Figs. 7.5 and 7.6). With lumbar spine bone mineral density the significant difference in percentage change persisted throughout the first 12 months post-burn and while statistical significance was not observed between the two groups at 18 and 24 months post-burn the differences trended in the same way as during the first year and the failure to demonstrate statistically significant differences between pamidronate and placebo groups at these time points may be attributable to the reduced number of subjects in each group and the large variability in percentage change from admission during that second year post-burn (see Fig. 7.7). More to the point, however, the significant difference in lumbar spine bone mineral density Z scores between the pamidronate and placebo subjects at 2 years post-burn (see Fig. 7.8) speaks to the persistence of the beneficial effect of acute pamidronate administration for at least 2 years on the bone mineral content and bone mineral density of burned children.
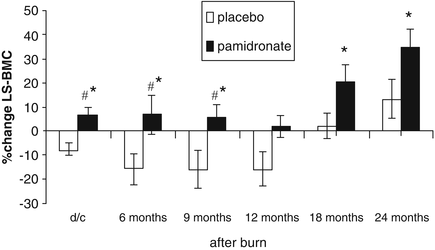

Fig. 7.5




Percent change in lumbar spine bone mineral content (d/c: hospital discharge). Values are presented as means and standard error of the means. Significant differences between placebo and pamidronate are designated by asterisk with p < 0.05. Significant time effect within each group when compared to changes at 24 months is designated by hash, with p < 0.005. (Reproduced from Przkora et al. [15] with permission of Elsevier.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


