Benign Changes of the Female Genital Tract 3 Glandular (Simple) Endometrial Hyperplasia
| 3 | Benign Changes of the Female Genital Tract |
Portio and Vagina
Physiological Changes
Cytolysis
Due to the activity of vaginal lactobacilli (Döderlein bacilli), the cytoplasm of intermediate cells lyses and glycogen is liberated. The sugar molecules derived from the glycogen are then metabolized into lactic acid. This creates an acidic environment and serves as a protective mechanism against potentially pathogenic microbes.
The physiological disintegration of squamous epithelial cells is called cytolysis. As the process requires glycogen-containing cells, it can only take place when the vaginal epithelium has developed at least the upper intermediate cell layer (Fig. 3.1) (411). No cytolysis is observed in an atrophic smear because parabasal cells do not contain glycogen (410). Superficial cells are protected from cytolysis because of their keratin-like cytoplasmic scaffold; hence, there is no cytolysis even when estrogen activity is high and the eosinophilia index is also high. (409).
Physiological cytolysis affects only the cytoplasm, not the nucleus (379). It is brought about by Döderlein bacilli, lactobacilli that exist only in the vagina. These bacilli are immobile rods of various lengths—though they exhibit a relatively uniform length within the same smear (227). Long piliform variants occasionally occur (Fig. 3.2). The bacilli completely or partially dissolve the cytoplasm of glycogen-containing intermediate cells, thus creating a fine granular protein precipitate that is sometimes misinterpreted as “coccal flora” by inexperienced examiners (Fig. 3.3). The bacilli then ferment the released glycogen to lactic acid. The resulting acidic environment (approximately pH 4) protects the epithelium against infections as it creates unfavorable growth conditions for foreign bacteria (410). Cytolysis therefore largely excludes the presence of pathogens from the vagina, although fungal infections are not affected by colonization of the vagina with lactobacilli.
If cytolysis is pronounced, most of the nuclei exist as naked nuclei and often show edematous swelling, although the chromatin structure is still well preserved and the nuclear envelope smooth. The enlarged nuclei pose a problem when cytolysis needs to be differentiated from dysplastic processes (Fig. 3.4). Topical application of an antibiotic will prevent cytolysis and may thus be advantageous when establishing the diagnosis (368).
The presence of a lactobacillus flora is not necessarily associated with the image of cytolysis in the vaginal smear. This is particularly true when the cells have been collected with a spatula, since this procedure predominately sets free cells derived from the superficial tissue layer where cytolysis caused by bacteria is not possible. By contrast, collecting the cells with a cotton swab yields cells from the vaginal secretion covering the portio vaginalis, and this essentially consists of defoliated cells that are in the process of undergoing cytolysis (322).
Degeneration
Unlike cytolysis, the degeneration of cells found in the cytological smear affects both cytoplasm and nucleus.
Nuclear changes:
- Nuclear swelling
- Karyolysis
- Karyorrhexis
- Karyopyknosis
- Hyperchomatic nuclear envelope
Cytoplasmic changes:
- Perinuclear halo
- Pseudoeosinophilia
- Vacuolation
- Hyalinization
- Structural changes
- Heterolysis
- Changes in cell shape
- Condensation of mucus
- Disturbed cell maturation

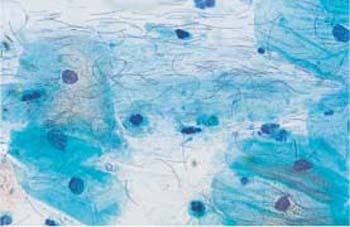
Fig. 3.2 Lactobacilli. A piliform, elongated morphological variant of these bacilli and vesicular cell nuclei are visible between the superficial cells. ×630.

Fig. 3.3 Cytolysis by lactobacilli. Vesicular, naked nuclei (→) and cytoplasmic debris, accompanied by a dust-like protein precipitate. ×630.
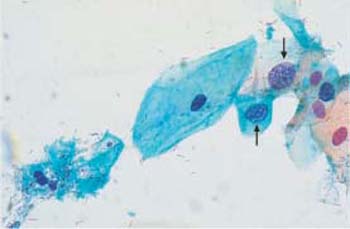
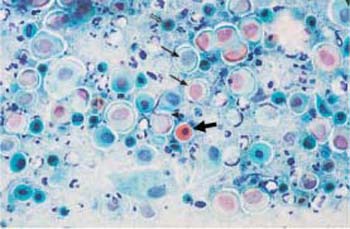
Fig. 3.5 Karyopyknosis. Nuclear degeneration of parabasal cells by both karyolysis and karyopyknosis, resulting in an increased (→) or decreased (⇒) nuclear size. The cytoplasm is often pale and pseudoeosinophilic, or it shows hyaline degeneration ( ) ×400.
) ×400.
Whereas cytolysis by lactobacilli is a sign of vitality of the squamous epithelium with well-preserved nuclei, degeneration is a process of cell death (necrosis) that is largely induced by the unfavorable environment of exfoliated cells. The absence of lactobacilli causes the pH to rise, thus leading almost instantly to the appearance of foreign bacteria (185). Even without a visible inflammatory reaction, this causes rapid cell death, with the loss of a normal chromatin structure being observed particularly in the less resistant parabasal and endocervical cells, but also in the more highly differentiated squamous epithelial cells and nonepithelial cells (93).
Nuclear Changes
- Nuclear swelling: Osmotic cell regulation ceases, causing water influx and nuclear swelling (Figs. 3.5–3.7). This may cause problems in distinguishing degeneration from dysplastic processes (160).
- Karyolysis: The formation of unstructured, hypochromatic, and enlarged nuclei is called karyolysis (Fig. 3.5).
- Karyopyknosis: Loss of water then causes the dead nuclei to shrink and become hyperchromatic; this is called nuclear condensation or karyopyknosis (Fig. 3.5) (178, 425).
- Karyorrhexis: The rupture of the nucleus into fragments is called karyorrhexis. The chromatin structure first becomes very coarse and then disintegrates, while gaps in the nuclear envelope result in a fragmented chromatin pattern (Fig. 3.6). The entire chromatin scaffold finally breaks up into many condensed and hyperchromatic fragments.
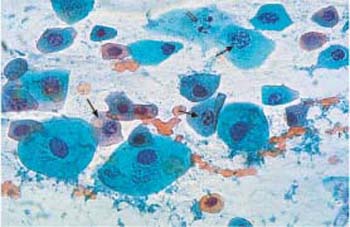
Fig. 3.6 Karyorrhexis. Nuclear degeneration by karyorrhexis, while the cytoplasm is still intact. The nuclei are hyperchromatic; they show a perforated nuclear envelope and a fragmented chromatin pattern (
 ). At the terminal stage, only some nuclear debris remains (⇒) ×630.
). At the terminal stage, only some nuclear debris remains (⇒) ×630.
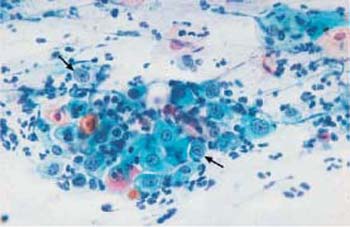
Fig. 3.7 Hyperchromatic nuclear envelopes. The smear shows atrophic vaginitis with partial activation and enlargement of the nuclei, and also formation of nucleoli. Also visible are degenerative changes of nuclei and cytoplasm, such as cytoplasmic signs of cytolysis and pseudoeosinophilia and distinct hyperchromatic nuclear envelopes (
 ). The background of the preparation indicates inflammation. ×400.
). The background of the preparation indicates inflammation. ×400.
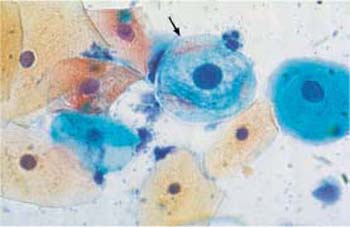
Fig. 3.8 Perinuclear halo. Cytoplasmic degeneration of a parabasal cell (
 ). The nucleus is already condensed, and the cytoplasm is amphophilic and shows a perinuclear halo. A still intact parabasal cell (on the right) and several superficial cells lie nearby. ×630.
). The nucleus is already condensed, and the cytoplasm is amphophilic and shows a perinuclear halo. A still intact parabasal cell (on the right) and several superficial cells lie nearby. ×630.
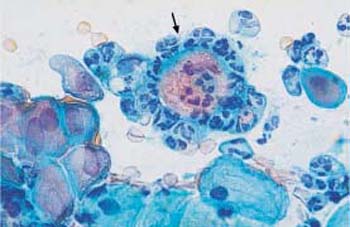
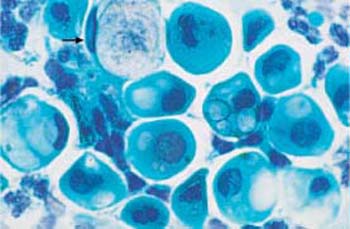
Fig. 3.10 Vacuolation. Cytoplasmic degeneration of parabasal cells by vacuolation, while the nuclei have already degenerated. The vacuoles are of variable size; if very large, they may push the nucleusto the margin of the cell (“signet-ring cell”) (
 ). ×1000.
). ×1000.
- Hyperchromatic nuclear envelope: Chromatin particles adhering to the inner nuclear membrane create the appearance of a hyperchromatic nuclear envelope (Fig. 3.7) (93).
Cytoplasmic changes
- Perinuclear halo: Shrinking of the nuclei leads to the formation of perinuclear halos (Fig. 3.8) (160).
- Pseudoeosinophilia: The degenerative changes eventually affect the cytoplasm as well; it becomes pseudoeosinophilic as a result of denaturation (Fig. 3.9) (368).
- Vacuolation: Water uptake leads to cell enlargement and formation of vacuoles (Fig. 3.10).
- Hyalinization: Condensation of the cytoplasm causes intense red staining, which is called hyalinization (Fig. 3.11) (356).
- Structural changes: Disintegration (or partial lysis) of the cytoplasm is often the result of structural changes. In some areas of the cell, the cytoplasm may exhibit complete chromophobia, although some rudimentary structures are still stained. The stained structures may appear as dots (“polka dot cells,” Fig. 3.12) or as ridges that form window-like gaps between them (“fenestrated cells,” Fig. 3.13) (212).
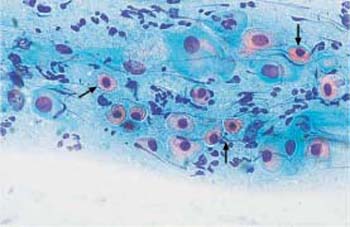
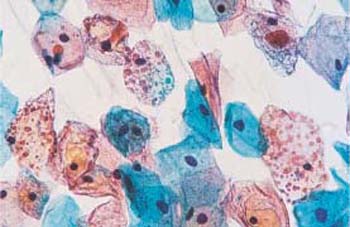
Fig. 3.12 Polka-dot cells. Cytoplasmic degeneration of superficial cells with partial disintegration resulting in a dotted pattern. Several normal intermediate cells are also present. ×500.
- Heterolysis: Lysis of the cytoplasm due to enzymes derived from bacteria other than Doderlein bacilli is called heterolysis (Fig. 3.14). Especially with atrophic cells, this may lead to confluence of several cells and formation of pseudosyncytial cells, known as cell cohesion (Fig. 3.15) (358). When the cytoplasm lyses completely, naked nuclei remain and often dominate the entire cell image.
- Changes in shape: Cell degeneration naturally affects atrophic smears, in particular, because parabasal cells are very sensitive to environmental factors. Elongation of the cytoplasm, possibly due to forces acting on the cell surface, leads to the formation of spindle-shaped cells (Fig. 3.16).
- Condensation of mucus: Chemotactic effects cause condensation of the mucus that covers the cells. This is probably responsible for the presence of blue blobs in the smears; these may be easily confused with enlarged naked nuclei with nucleoli (Fig. 3.17) (1)
- Disturbed cell maturation: Severely degenerated smears occasionally exhibit signs of a disturbance of squamous epithelial maturation, showing cells with hugely enlarged nuclei (Fig. 3.18). These are most likely the result of spontaneous gene mutation in old age, causing polyploidization of individual nuclei. Estrogen treatment may hasten the maturation of some parabasal cells, thus creating miniature superficial cells (Fig. 3.19) (416).
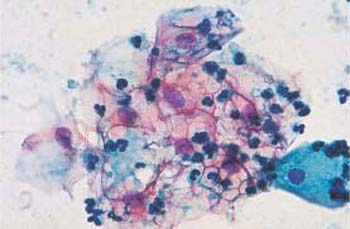
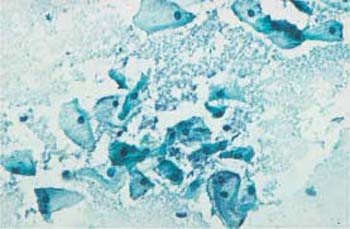
Fig. 3.14 Image of heterolysis. Infection with Gardnerella causes the cytoplasm of intermediate cells to disintegrate without simultaneously inducing an inflammatory reaction. ×250.
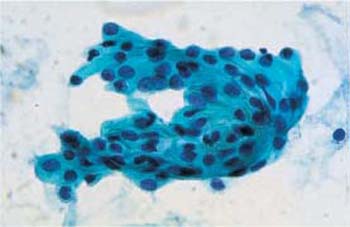
Fig. 3.15 Atrophic cell cohesion. Pseudosyncytial cell sheet formed by parabasal cells. ×630.
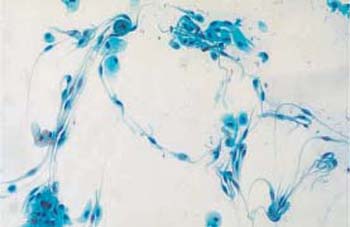
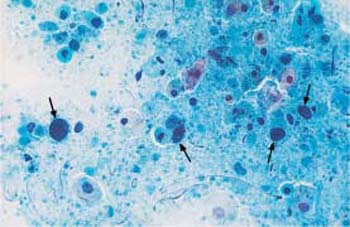
Fig. 3.17 Blue blobs. Atrophic cell image with degenerating parabasal cells. Some mucus has condensed on top of individual parabasal cells (
 ), presumably due to chemotactic effects, thus making them look hyperchromatic. They are therefore easily confused with enlarged nuclei of malignant cells. ×400.
), presumably due to chemotactic effects, thus making them look hyperchromatic. They are therefore easily confused with enlarged nuclei of malignant cells. ×400.
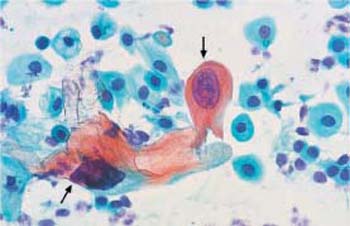
Fig. 3.18 Polyploidization. Atrophic smear in old age. Hugely enlarged and hyperchromatic nuclei often lead to confusion with abnormal nuclei (
 ) As they often coexist with degenerative nuclear changes, the chromatin structure cannot be assessed. ×400.
) As they often coexist with degenerative nuclear changes, the chromatin structure cannot be assessed. ×400.

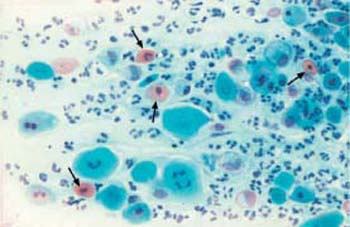
Fig. 3.20 Atrophic vaginitis. The degenerated parabasal cells are accompanied by a cell image of chronic inflammation. Granulocytes, lymphocytes, and histiocytes, as well as hyaline, degenerated parabasal cells
 result in a colorful cell image. ×250.
result in a colorful cell image. ×250.
Atrophic vaginitis. The atrophic vaginal epithelium is less resistant to bacterial or fungal superinfections and also to mechanical irritations and injuries. Inflammatory superinfection leads to atrophic vaginitis (Fig. 3.20). To prevent this from happening, systemic—or at least topical—estrogen replacement after the menopause is advantageous (194) (see also p. 124).
Fig. 3.22a, b Squeezed cells. These are the result of improper methods of smear collection.
a Cells with longitudinally flattened cytoplasm. ×320.

Technical Factors
Cell damage due to technical factors may be caused by simple errors during collection and processing of the material, or by complex iatrogenic cellular reactions such as those following radiation or chemotherapy.
Sampling Method
Improper collection of samples from the cervix may be the reason why insufficient numbers of cells have been harvested, or why the number of squamous or columnar epithelial cells is too low for an assessment. The swab technique therefore greatly influences the assessment of a smear (Fig. 3.21).
Furthermore, only instruments that are suitable for harvesting sufficient amounts of representative cell material from the collection site should be used.
Smear Technique
- Squeezing of cells caused by a very swift smear technique may lead to longitudinal stretching of both cytoplasm and nuclei. This creates spindle-shaped cells, which are often confused with abnormal cells (Fig. 3.22a, b).
- Superposition of cells may lead to misinterpretations of the correct size of cells and nuclei (Fig. 3.23).
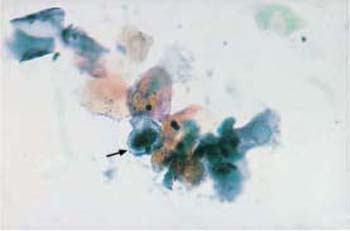
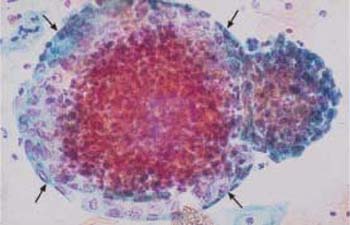
Fig. 3.23 Superposition of cells. When lying on top of each other, cells of variable size appear as pseudohyperchromatic cells due to the additive effects of stained material, thus mimicking nuclear atypia ( ) ×250.
) ×250.
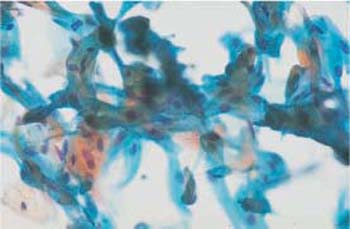
Fig. 3.24 Disorganized arrangement of cell material. This is the result of improper methods of smear collection using circular movements of the cotton swab. ×400.
When the material is smeared in an irregular fashion—for example, in circles or in a zigzag pattern—squamous and columnar epithelial cells become mixed up and their ectocervical or endocervical origin is no longer recognizable (Fig. 3.24).
Displacement of Cells
Contamination of smears with displaced cells normally occurs when samples are collected during menstruation. Although a lactobacillus flora may be present, the smear has an inflammatory appearance when plenty of leukocytes, histiocytes, endometrial cells, mucus, and blood is present (see Fig. 2.28, p. 23). These cells may also occur during midcycle and in the absence of macroscopically relevant breakthrough bleeding—in particular, when the patient is taking one of the low-dose ovulation inhibitors that are preferred today. In these patients, the epithelium often shows a low degree of differentiation or is even atrophic (see Fig. 2.30, p. 25) (288). Groups of endometrial cells appearing at the end of menstruation, or even during the first half of the cycle, are called exodus. They appear as spherical cell clusters with a stromal center and an epithelial periphery (Fig. 3.25) (277).
Anucleated horn cells found in the vaginal smear have usually been displaced and are not typical for the collection site; they originate either from the genitals of the sexual partner, or the hands or vulva of the patient (Fig. 3.26) (244). In case of a prolapsed uterus, or when the patient uses a vaginal diaphragm, these cells may be the result of epidermization caused by pressure (Fig. 3.27) (368).
In rare cases, the staining solution may have been contaminated externally, thus transferring certain fungal, plant, or animal parts to the smear (33):
- The fungus Alternaria is characterized by segmented conidiospores that resemble snowshoes or beer barrels (Figs. 3.28, 3.29).
- The mold Aspergillus is characterized by Y-shaped ramifications and typical conidio-phores (Fig. 3.30a, b).
- The fungus Geotricum forms regular, sharply defined geometrical structures (Figs. 3.31, 3.32).
- Plant cells (Figs. 3.33-35) and cerci of beetles (Fig. 3.36a, b) may be present.
- Oxyurid eggs (Fig. 3.37) and pubic lice (Fig. 3.38) are found in exceptional cases, due to contamination of the medium when moving the swab across the hair of the vulva.
Some artifacts are not easy to identify (Fig. 3.39a, b). The accidental transfer of cells from one microscope slide to another is very rare, but it may have grave consequences in case of malignant cells (Fig. 3.40) (321).
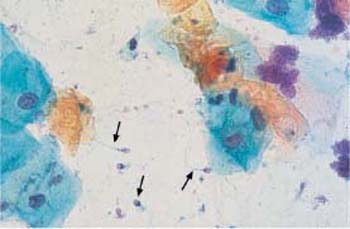
Fig. 3.27 Orthokeratotic cell sheet. Here, the horn cells are due to a local disturbance of differentiation leading to epidermization of the portio in response to a vaginal diaphragm. Numerous parabasal cells are visible at the top. ×100.
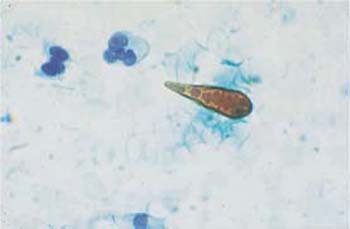
Fig. 3.28 The fungus Alternaria. Typical segmentation and snow-shoe appearance. ×1000.
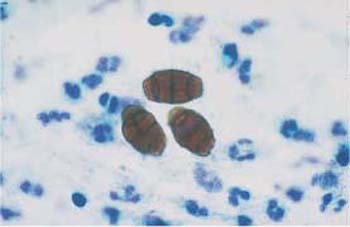
Fig. 3.29 The fungus Alternaria. Typical segmentation and beer-barrel appearance. ×1000.
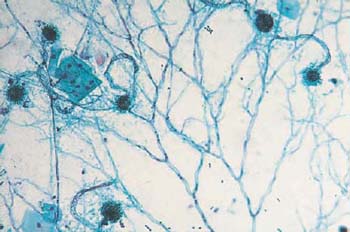
Fig. 3.30a, b The fungus Aspergillus.
a Typical Y-shaped ramifications and terminal conidiophores. ×160.
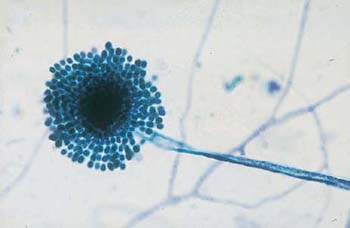
b A typical conidiophore consisting of a dense core and a corona of blastospores. ×500.
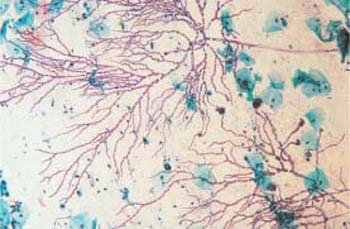
Fig. 3.31 The fungus Geotricum. Regular geometric ramifications. ×120.

Fig. 3.32 The fungus Geotricum. Regular geometric ramifications and sharp marginal contours. ×200.
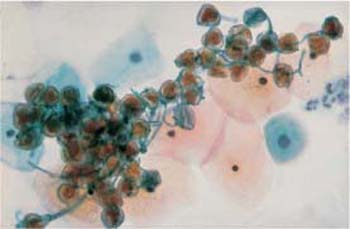
Fig. 3.33 Pollen. A loose aggregation of brownstaining, round structures. Several superficial and intermediate cells are visible in the background. ×400.
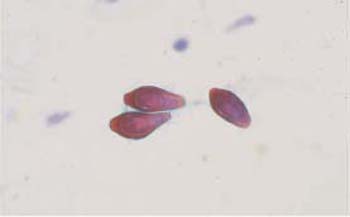
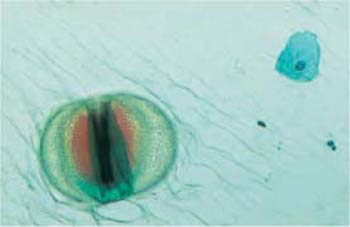
Fig. 3.35 The diatom Cosmarium. A large segmented imicellular alga with chlorophyll. ×250.

Fig. 3.36a, b Cerci of the museum beetle (Anthrenus museorum).
a ×200.

b A single cercus is surrounded by several superficial cells. ×250.
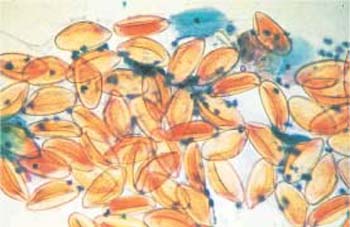
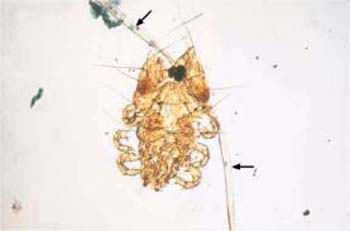
Fig. 3.38 Pediculosis of the vulva. Retrograde transmission of a small pubic louse, including the pubic hair ( ) onto which it clings. ×100.
) onto which it clings. ×100.
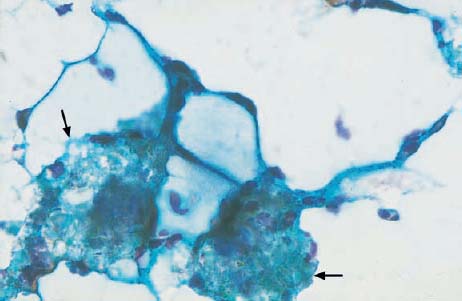
b Artifact consisting of mucous vacuoles and ointment residues ( ). ×630.
). ×630.
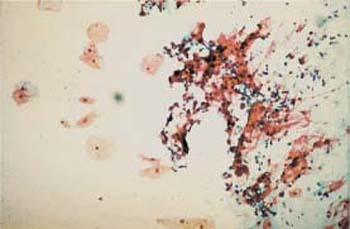
Fig. 3.40 A cluster of contaminating cancer cells (right half of image). Cancer cells of a patient with cervical carcinoma have been accidentally transferred from her microscope slide onto that of a young healthy woman (a virgin), presumably during fixation within the same cuvette or during the staining procedure. ×100.
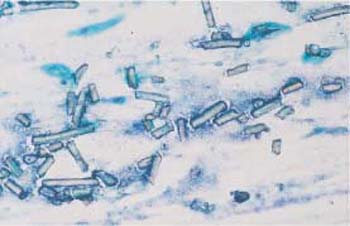
Fig. 3.42 Plastic fibers. The cervical smear is obscured by sharply defined plastic fibers that probably originate from textiles. ×200.
Obscuring of Cells
Assessment of the smear may be severely hampered if the representative cells are obscured by foreign cells, mucus, or artifacts. These may include talcum, starch granules, textile fibers (most often the remains of cotton swabs or tampons), as well as residues of ointments or oils derived from intravaginal application.
- Admixture of mucus: Mucus often stains cyanophilic or blue-green, less often eosinophilic.
- Starch granules: These have characteristic structures and birefringent properties (Fig. 3.41).
- Plastic fibers: These are sharply defined and do not disintegrate (Fig. 3.42).
- Natural fibers: These show signs of disintegration and may exhibit leukocytic infiltration and even cell nuclei (Fig. 3.43).
- Ointments and oils: The fat component of these has usually been completely removed by alcohol during the staining procedure, but other components may be detected as blue-green, homogenous artifacts (Fig. 3.44) (246). These amorphous substances are sometimes resorbed by phagocytic cells (Fig. 3.45).
- Blood: Admixtures of blood appear pleomorphic. Erythrocytes may be still intact, or may have disintegrated to form lysed blood (Fig. 3.46). When lysed blood contaminates adjacent areas of the smear, it causes pseudoeosinophilia at these sites (360). Menstrual blood is subjected to fibrinolysis by an enzyme locally produced in the mucosa; it does not coagulate (211). For this reason, fibrin thrombi are not detected under normal circumstances, but they may develop after injury or malignant erosion of blood vessels (Fig. 3.47). Hemosiderin, the intracellular storage form of iron, has the appearance of eosinophilic or blue-green granules (Fig.3.48a, b) (368). Large histiocytes that incorporate blood components are called siderophages(Fig. 3.49).

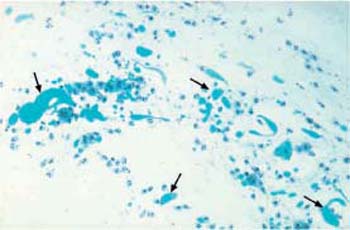
Fig. 3.44 Ointment residues. After application of intravaginal medication, the ointment remains in the form of blue-green amorphous structures (
 ) surrounded by histiocytic cells. ×100.
) surrounded by histiocytic cells. ×100.
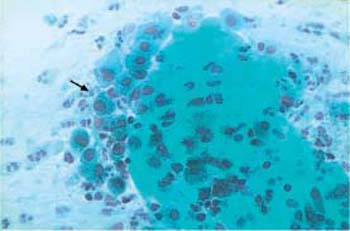
Fig. 3.45 Ointment components. The ointment is being resorbed by surrounding macrophages ( ). ×250.
). ×250.
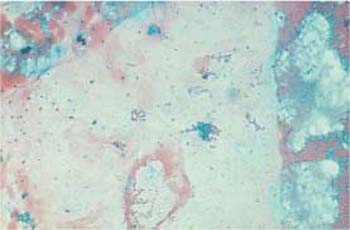
Fig. 3.46 Blood obscuring the cervical smear. The blood is partly corpuscular (erythrocytes), partly lysed. ×250.
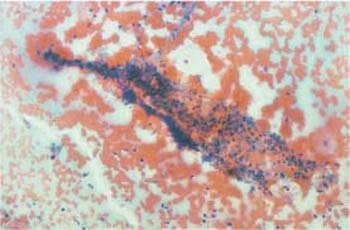
Fig. 3.47 Fibrin thrombus. Leukocytic infiltration of a thrombus, surrounded by intact erythrocytes and caused by vascular erosion due to invasive cervical carcinoma. ×100.
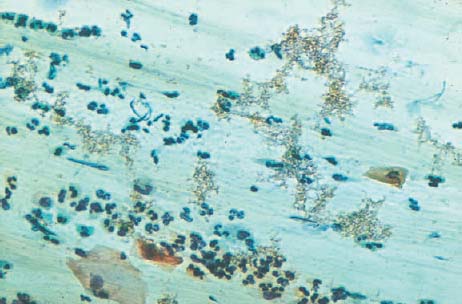
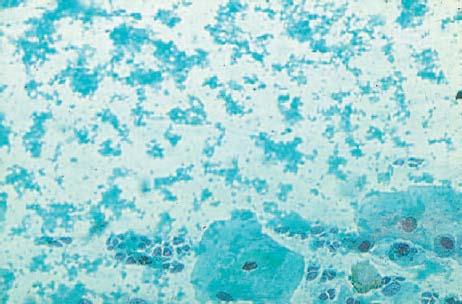
b Blue-green hemosiderin.
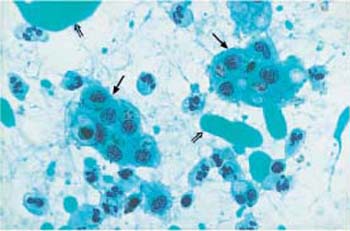
Fig. 3.49 Siderophages. Loose clusters of macrophages ( ) that have incorporated blood components. Leukocytes and blue-green ointment residues (⇒) are visible nearby. ×400.
) that have incorporated blood components. Leukocytes and blue-green ointment residues (⇒) are visible nearby. ×400.
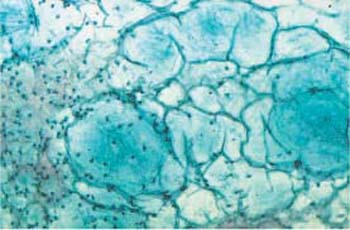
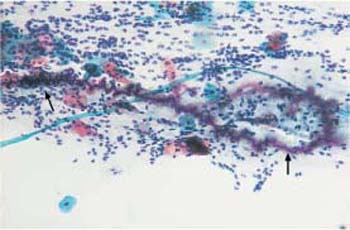
Fig. 3.51 Curschmann spiral. The coiled mucinous fiber ( ) was probably created by the action of ciliary cells. ×160.
) was probably created by the action of ciliary cells. ×160.
Fixation Errors
Desiccation of the cells due to delayed fixation or dilution of the fixative frequently causes discoloration of the smear, pseudoeosinophilia, and nuclear degeneration(Figs. 3.52, 3.53) (368). The artifacts may be misinterpreted as keratinized cells or enlarged nuclei. The changes may affect only parts of the smear, particularly marginal areas, either because the entire smear was not homogeneously covered by spray fixative, or because the microscopic slide was not completely immersed in the fixation dish.
Staining Errors
Discoloration of the smear may be caused by inferior batches of stain, wrong staining periods, or excessive use of the same staining series (368).
On the other hand, prolonged staining with certain solutions, or the use of unsuitable batches, may result in overstaining of the cytoplasm, thus making it difficult to distinguish between cytoplasm and nucleus (Fig. 3.54). Central pseudoeosinophilia of cell clusters is also typical for overstained preparations (Fig. 3.55) (368).
Occasionally, hematoxylin may precipitate as a result of insufficient washing during the staining procedure; this is called hematoxylin pigment (Fig. 3.56) (368).
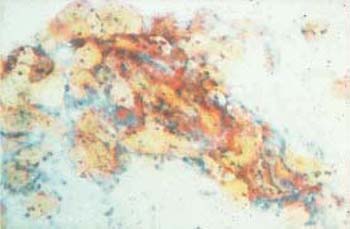
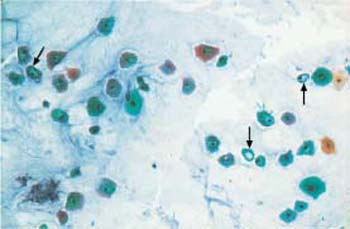
Fig. 3.53 Fixation artifacts. Caused by desiccation, these artifacts mimic cytoplasmic vacuolation or koilocytosis ( ) ×100.
) ×100.
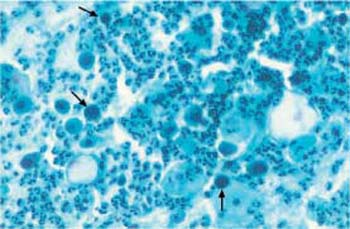
Fig. 3.54 Overstaining. Caused by staining error. The nuclei of parabasal cells are difficult to distinguish from the cytoplasm ( ). ×250.
). ×250.
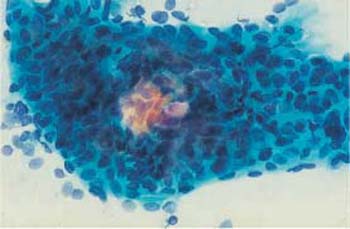
Fig. 3.55 Pseudoeosinophilia. The center of the dense cell cluster is incompletely stained. ×400.
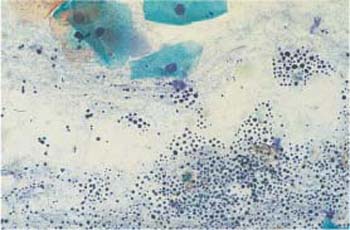
Fig. 3.56 Hematoxylin pigment. The precipitation is probably due to incomplete washing during the staining procedure. Superficial cells are visible at the top. ×400.
Mounting Errors
The cornflake artifact is due to the formation of brownish, round granules on top of squamous epithelial cells, thus severely hampering assessment of the smear (Fig. 3.57a, b). This phenomenon probably results from the inclusion of air during the mounting process and is caused by water evaporation due to insufficient dehydration (368). The dark coloration is due to the interference of light reflected between air, mounting medium, and cell surface. This frequently observed phenomenon is therefore explained by exposure to water and air during improper or delayed mounting (101).
Assessment is not possible with large inclusions of air, or when the wrong side of the slide has been mounted, because the optical parameters have changed. Proper remounting of the smear is required (Figs. 3.58, 3.59).
Confusion of Material
Confusion of patients’ names, resulting in the wrong assignment of cell material, may occur at the sender’s end or in the cytological laboratory. The error is usually not recognized and is of no consequence as long as the cytological findings are negative. If, however, a mix-up is suspected and the findings are positive, the patients involved need to be recalled to the originating doctor’s office or clinic for the smear preparation to be repeated.
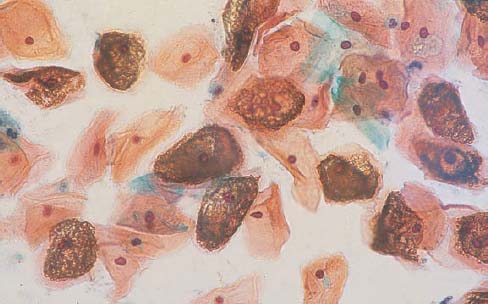
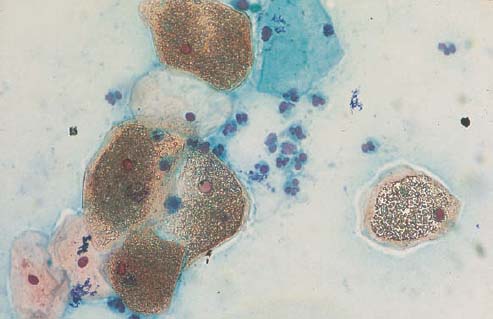
b The cornflake pattern overlying the cells may severely hamper the diagnosis. ×400.
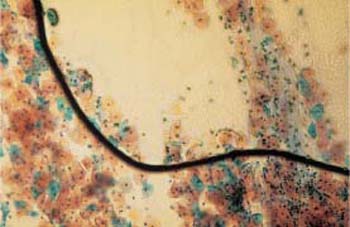
Fig. 3.58 Inclusion of air(upper right half of image). This often changes the cell image to such an extent that expert assessment of the smear is no longer possible. Remounting may help. ×100.
Radiation
The effects of radiation occur less often with the glandular epithelium than with the readily regenerating squamous epithelium. Immature cells, such as parabasal cells and metaplastic cells, are especially affected (Fig. 3.60a–d) (38). Since ionizing radiation attacks the sensitive nucleic acids, it irreversibly damages the genetic material of the cells. For this reason, effects of radiation can still be recognized in cytological smears for years and even decades after radiation therapy (388). Radiation always leads to changes in the overall image of the smear, which shows initially acute, and later chronic, inflammatory reactions (121).
Initially, the changes affect mainly the immature cells, but after only a few weeks they are also pronounced in all other layers of the squamous epithelium (Figs. 3.61 a, b; 3.62a, b; 3.63a, b) (388). These changes consist mainly of irregular vacuolation as well as phagocytosis and cannibalism, a process leading to the formation of “bird’s eye cells.” The changes also include macrocytosis, leukotaxis, and changes in color and structure, such as pseudoeosinophilia, amphophilia, bizarre cytoplasmic formations, and spindle-shaped cells.
At the same time, the size of the nuclei increases without significantly shifting the nucleocytoplasmic ratio, thus resulting in proportional nuclear enlargement. The nuclear structure may become hyperchromatic, although without any atypia. Pronounced degenerative nuclear changes usually occur in the form of karoylysis, karyorrhexis, or karyopyknosis. Binuclear and polynuclear cells are common, and enlarged nucleoli as a sign of the ongoing epithelial regeneration are also observed.
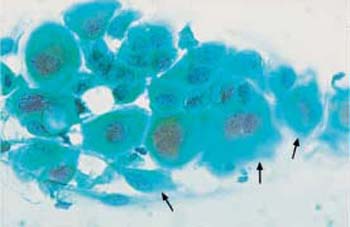
Fig. 3.60a–d Acute radiation effects.
a Parabasal cells with enlarged and degenerated nuclei, amphophilic cytoplasm, and indistinct cell borders ( ). ×400.
). ×400.
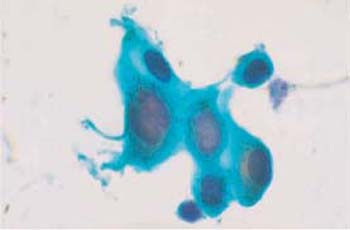
b Small intermediate cells with blurred cell borders and enlarged, degenerated nuclei. ×1000.
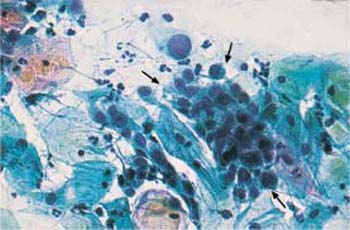
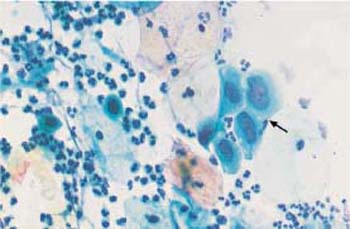
c A cluster of endocervical columnar epithelial cells ( ) with enlarged, hyperchromatic nuclei. Some nuclei show distinct chromocenters. Superficial and intermediate cells are visible nearby. ×250.
) with enlarged, hyperchromatic nuclei. Some nuclei show distinct chromocenters. Superficial and intermediate cells are visible nearby. ×250.
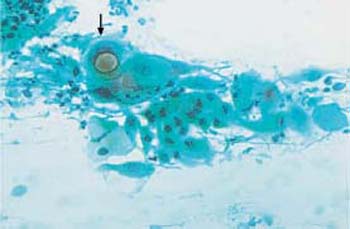
d A cluster of endocervical columnar epithelial cells ( ) with enlarged, hyperchromatic nuclei. Nuclear and cytoplasmic structures are blurred. Superficial cells are visible nearby. The background of the preparations indicates inflammation. ×250.
) with enlarged, hyperchromatic nuclei. Nuclear and cytoplasmic structures are blurred. Superficial cells are visible nearby. The background of the preparations indicates inflammation. ×250.
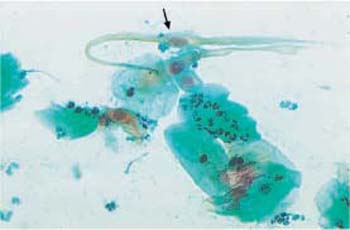
b Appearance of spindle-shaped cells. The nuclei are degenerated and hypochromatic ( ) ×250.
) ×250.
Folic Acid Deficiency
Deficiency of vitamin B12 and/or folic acid may occur during pregnancy and in association with certain forms of anemia. It has a negative effect on all cells because these substances are required for DNA synthesis (258). A characteristic sign is macrocytosis with proportional enlargement of cells and nuclei of the squamous epithelium, and binuclear cells and cytoplasmic vacuolation are also found occasionally (Fig. 3.64).
Cytostatic Treatment
Similar changes to those of folic acid deficiency are observed after treatment with cytostatic agents, since many of these substances function as folic acid antagonists (368,392). The resulting changes of the cell image are usually even more pronounced than in folic acid deficiency (Fig. 3.65a). The nuclei may be hyperchromatic and polymorphic, and partial nuclear condensation is occasionally observed (Fig. 3.65b).
Surgical Sequelae
Within the first 4 weeks after an intravaginal surgical intervention, such as conization or hysterectomy, granulation tissue usually develops in the area of the surgical wound (81). Cytologically, this tissue consists of squamous and endocervical epithelia with inflammatory changes, regenerating epithelium, leukocytes, histiocytes, fibroblasts, and protein-rich cell debris (Fig. 3.66).
b Macrocytosis, cannibalism, and phagocytosis resulting in so-called bird’s eye cells ( )630×.
)630×.
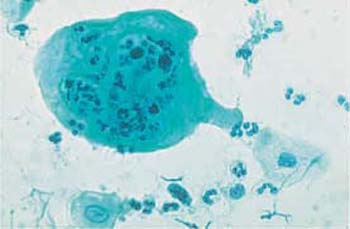
Fig. 3.63a, b Polynuclear giant cells. Caused by radiation effect.
a Giant cell with phagocytosed leukocytes. ×400.
b Giant cell with vacuolation, pseudoeosinophilia, and indistinct cell borders. ×630.
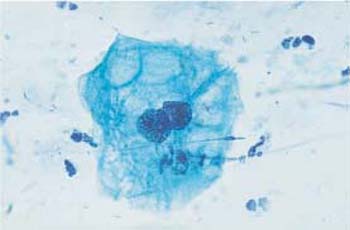
Fig. 3.64 Folic acid deficiency. Macrocytosis and cytoplasmic vacuolation of a binuclear cell. ×630.
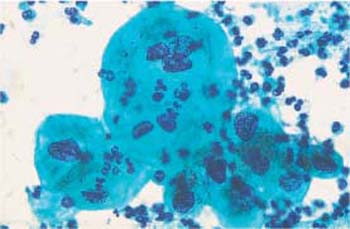
Fig. 3.65a, b Effect of chemotherapy.
a Polynuclear cells with proportional nuclear enlargement and hyperchromatic, polymorphic nuclei. ×630.
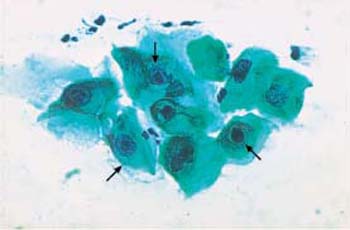
b Cells with hyperchromatic, polymorphic nuclei and central nuclear condensation ( ) ×400.
) ×400.
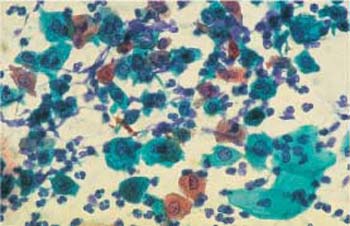
Fig. 3.66 Granulomatous postoperative inflammation. Cell image with polymorphic cells and nuclei. The background of the preparation indicates inflammation. ×400.
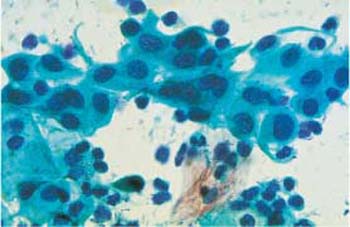
Fig. 3.67 Intrauterine device. A loose cluster of immature metaplastic cells, with an inflammatory cell image caused by the presence of an IUD. ×630.
As a late sequel of hysterectomy, columnar epithelial cells may be present in the smear if the endocervix has not been completely removed, or if portions of the uterine tubes have been caught in the vaginal blind sac, a condition referred to as tubal prolapse (81).
Intrauterine Devices
Patients using an intrauterine device (IUD) often show reactive changes in the smear image. These include nuclear enlargement and vacuolation in the endocervical columnar epithelium, presence of metaplastic cells (Fig. 3.67) and endometrial cells, inflammatory cells in the background, and, in particular, signs of actinomycosis (78) (see also p. 92).
However, it is only this combination of changes that is characteristic for the presence of an IUD, not any of the individual changes. The chronic state of irritation caused by the foreign body seems to promote colonization of the cervix by pathogens.
Abnormal Microbiology
Facultative pathogenic microorganisms may be detected in the cervical smear, sometimes also together with isolated damaged cells, without inducing an inflammatory reaction visible in the overall cell image. Whether an inflammatory reaction occurs depends on the pathogen count and the aggressiveness of the organism, but, most importantly, on the body’s state of immunity.
Most of the time, these microorganisms exist in a parasitoid, saprophytic state. They are nonaggressive but may lead to degenerative changes of cells (314). Many pathogens may be identified on the basis of the characteristic cytological changes they induce in the smear. The physiological lactobacillus flora is present in less than one third of all women.
Mixed Flora
A mixed vaginal flora consists mostly of cocci and rods, and the latter may temporarily include lactobacilli (Fig. 3.68a, b). As some bacteria measure only a few tenth of a micrometer, the 100× magnification commonly used for screening is not sufficient for microscopic differentiation of the flora. Identification requires a 400× magnification, although the exact assignment and differentiation of bacteria are not possible in a Papanicolaou smear (185). For proper identification, special staining procedures or cultures are required.
Cocci
Sometimes there is a pure coccal flora consisting of evenly spread, isolated, and punctiform bacteria. Such an infection frequently causes an increase in karyopyknosis, eosinophilia, or even pseudokeratinization of the squamous epithelial cells (Fig. 3.69a, b) (368). An inexperienced examiner may misinterpret these changes as the effect of increased follicular hormone activity, or a sign of HPV infection, or keratinizing dysplasia.
Gardnerella
A Gardnerella flora is relatively common and sometimes difficult to distinguish from a coccal flora (74). It occurs predominantly when the vaginal epithelium is highly differentiated (98).
The bacterium was formerly called Haemophilus vaginalis. Clinically, the infection is accompanied by a characteristic fishy odor caused by the release of amines. If there are signs of inflammation, this is referred to as bacterial vaginosis (80). Addition of potassium hydroxide solution to the vaginal discharge intensifies the odor, which is the diagnostic principle of the amine test (79).
The bacterial lawn in the cytological smear resembles dust-like particles with a tendency to accumulate like sand dunes. In addition, there are many squamous epithelial cells that are densely covered with bacteria due to a chemotactic effect; these are called “clue cells” (198). These changes are detected in native smears by phase contrast microscopy (Fig. 3.70) as well as in stained preparations (Figs. 3.71, 3.72 a, b).
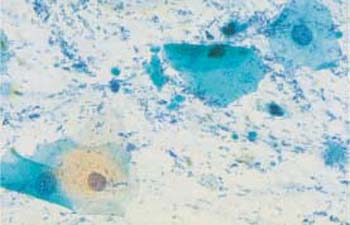
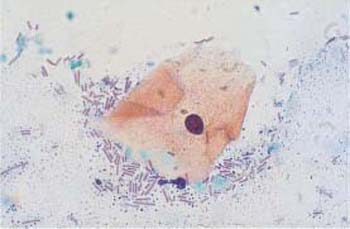
b Cocci and rods surrounding an eosinophilic superficial cell with pyknotic nucleus. ×1000.
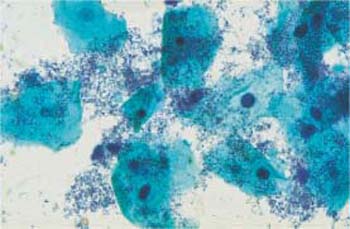
Fig. 3.69a, b Coccal flora.
a Punctiform bacteria. No inflammatory reaction of the squamous epithelium. ×630.
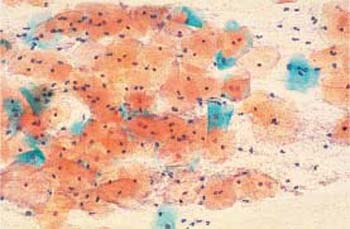
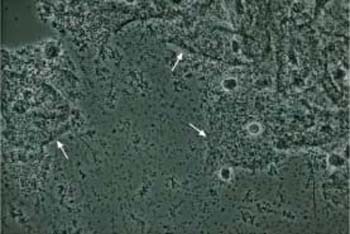
Fig. 3.70 Clue cells associated with Gardnerella infection. Phase contrast image of superficial cells overlaid by a dense bacterial lawn ( ). The background shows the typical image of dustlike particles. ×630.
). The background shows the typical image of dustlike particles. ×630.
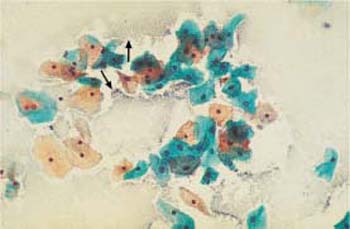
Fig. 3.71 Gardnerella flora. Typical sand-dune phenomenon ( ). No inflammatory reaction of the squamous epithelium. ×250.
). No inflammatory reaction of the squamous epithelium. ×250.
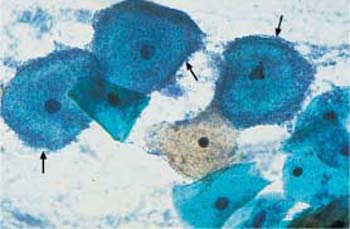
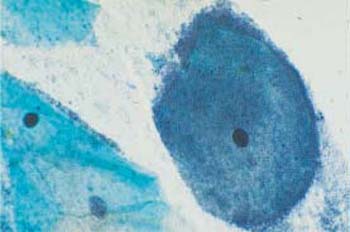
b Dense concentration of bacteria on an intermediate cell. ×1000.
Chlamydia
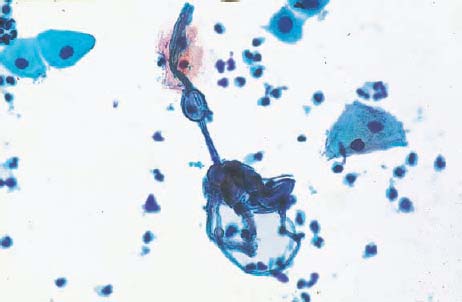
Bacteria of the genus Chlamydia are very small and therefore difficult to recognize. Nevertheless, they may be detected in cervical smears by means of special methods (230). Because of their high pathogenicity, a special screening test is carried out during early pregnancy in the context of maternity care to ensure timely treatment of any infection (253). Chlamydia infection represents one of the most common causes of sterility, since it often leads to ascending inflammation with involvement of the uterine tubes, thus causing salpingitis (7). In the course of inflammation, scarring may block tubal patency.
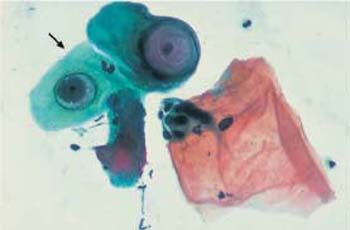
Both the presence and the reproduction of Chlamydia depend on an energy-rich metabolism. These bacteria can therefore only survive as intracellular parasites. They preferentially infect parabasal cells, immature metaplastic cells, endocervical cells, and urothelial cells (99). In these cells, the pathogen forms cytoplasmic vacuoles with diffuse borders (inclusion bodies) of variable size without affecting the nuclei (Fig. 3.73a–c) (118). The reproductive cycle takes place within these vacuoles, leading to the formation of elementary bodies, which show a bluish to reddish staining (352). Once the host cell is destroyed, the bacteria are released and may become infectious again. Detection of epithelial cells suspected to contain Chlamydia is too nonspecific to allow the assumption of an actual infection. At the most, such cells may be the reason for employing special detection methods using monoclonal antibodies to Chlamydia.
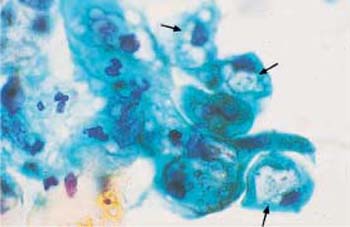

b Vacuoles of variable size and inclusion bodies within a parabasal cell ( ). ×1000.
). ×1000.
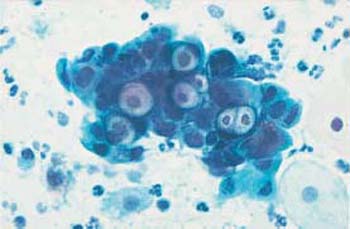
c A cluster of endocervical cells with vacuoles of variable size, indistinct margins, and eosinophilic inclusion bodies. The background of the preparation indicates inflammation. ×400.
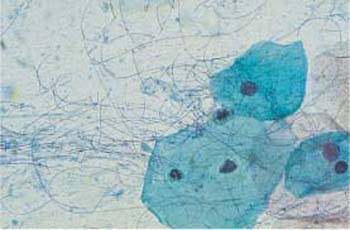
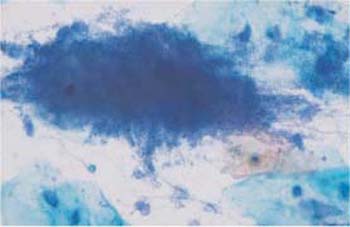
Fig. 3.75 Actinomycosis. Bacterial lawn showing a dark, central concentration and peripheral filaments. ×360.
Leptothrix
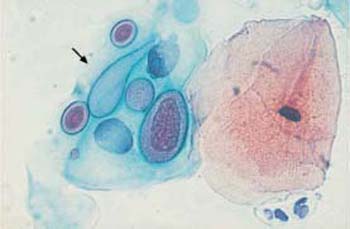
These apathogenic filamentous bacteria are thin, piliform microbes measuring up to 100 pm in length and mostly forming curves or loops (Fig. 3.74) (55). In comparison with other bacteria, they are rather rare, although they tend to appear together with trichomonads.
Actinomycetes
Actinomycosis, an infection caused by actinomycetes, is observed in 10-20% of all IUD users (117). As a rule, the background of the smear indicates a severe inflammatory reaction, although the pathogenicity of this infection seems to be minor (81).
Actinomycosis is a bacterial infection and is recognized by dirty-looking, moldlike, filamentous aggregations of various sizes. Frequently, these mycelial structures have a central condensation from where fine, irregular filaments extend to the periphery. They are also called actinomycotic drusen, sulfur bodies, or Gupta bodies, and consist of bacteria, proteins, and polysaccharides (Fig. 3.75) (117).
Candida
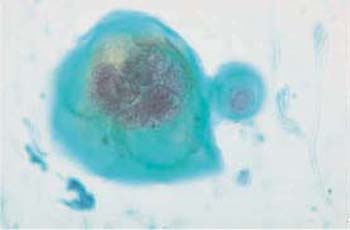
The yeast Candida albicans is frequently detected in vaginal smears. This is especially true following antibiotic therapy, in diabetic patients with elevated glycosuria, and occasionally after taking high doses of estrogen (386). Macroscopically, candidiasis is often associated with a whitish coat of the portio vaginalis (Fig. 3.76).
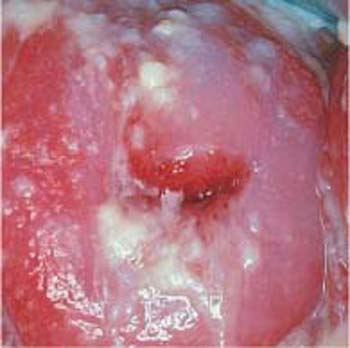
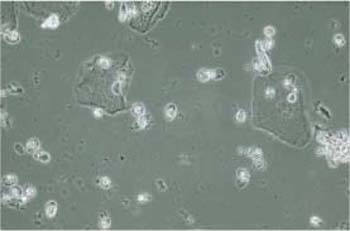
Fig. 3.77 Budding cells. Phase contrast image of vaginal candidiasis. In addition to some superficial cells, numerous brightly reflecting, oval budding cells are present. ×630.
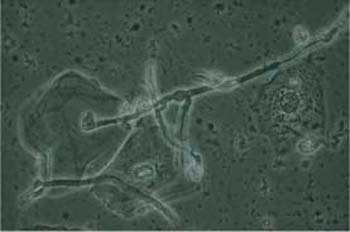
The detection of this fungus is much easier in wet mounts than in stained preparations because the pathogen shows higher contrast when viewed by phase contrast microscopy (Figs. 3.77, 3.78) (40). The exact identification of different fungal types in the cytological smear is not possible; it requires special culture media.
In stained preparations, fungal infections often yield a diffuse, milky background and are associated with pseudoeosinophilia and partial disintegration of individual squamous epithelial cells in the form of polkadot cells or fenestrated cells (Fig. 3.79, and see Figs. 3.12, 3.13 on pp. 60, 61).
The yeast either forms oval budding cells (blastospores), or develops tubular hyphae (pseudomycelia), both of which are either eosinophilic or do not stain at all (Figs. 3.80a–c, 3.81, 3.82) (170). Budding yeast cells measure about 5um, thus being slightly smaller than erythrocytes; they often show budding protrusions in a “mother-and-child” configuration. By contrast, hyphae may reach a length of 100 um and more. They consist of characteristic, double-contoured tubes, which are segmented and branched and show irregular, bamboo-like constrictions (Figs. 3.78, 3.82). These hyphae often form complex networks. Degenerating hyphae lose their double contours and are then difficult to distinguish from threads of mucus or elongated leukocytes (139).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 ) begin to phagocytose the dead cell. ×630.
) begin to phagocytose the dead cell. ×630. ) ×630.
) ×630. ) ×400.
) ×400. ) ×400.
) ×400. ), suggesting that the horn cells are most likely derived from the partner. ×400.
), suggesting that the horn cells are most likely derived from the partner. ×400. ). The nuclei are degenerated. ×250.
). The nuclei are degenerated. ×250. ). ×630.
). ×630. ). ×630.
). ×630. ). ×1000.
). ×1000.