7.3 Organisms
In most Western countries, the most common organisms causing neonatal bacterial meningitis are GBS, E. coli, other Gram-negative bacilli, S. pneumoniae and Listeria1,2,21,39 (Table 7.1). In a US study which only reported cases of meningitis in infants <;2 months due to GBS, H. influenzae, S. pneumoniae, N. meningitidis and Listeria, GBS caused 86.1% of cases and Listeria 5%.39 In a French study,21 GBS (59%) and E. coli (28%) were by far the commonest cause of neonatal bacterial meningitis, followed by Gram-negative bacilli other than E. coli (4%), other streptococci (4%), N. meningitidis (3%), and Listeria monocytogenes (1.5%). In early-onset meningitis, GBS caused 77% of cases and E. coli 18%, while in late-onset meningitis GBS caused 50% and E. coli 33%. However, E. coli (45%) was more common than GBS (32%) in pre-term infants and especially in very pre-term infants (54%).21
Table 7.1 Organisms causing neonatal meningitis in Western countriesa.
| Frequency of occurrence | Organisms |
| Common (>10%)* | Group B streptococcus (GBS) Escherichia coli |
| Uncommon (1–10%) | Other Gram-negative enteric bacilli Listeria monocytogenes Streptococcus pneumoniae Enterococci (faecal streptococci) Other streptococci |
| Rare (<;1%) | Coagulase-negative staphylococci Staphylococcus aureus Haemophilus influenzae (usually untypeable) Neisseria meningitidis Anaerobes |
| aThe exact proportions vary from country to country, with time, and with the use of intrapartum chemoprophylaxis. | |
The incidence of GBS in developing countries varies. There were no cases of GBS meningitis in the case series from the Gambia, Ethiopia, Niger, Nigeria, Kenya, the Philippines, Papua New Guinea, Jordan, Saudi Arabia and Panama, summarized in a systematic review.3 However, GBS was the most commonly reported organism causing meningitis in case series from Qatar, the United Arab Emirates, Malawi, Zimbabwe, South Africa and Trinidad, West Indies and was an important cause in case series from Thailand and Kenya.3 It is difficult to know if these differences are because of variations in laboratory techniques or represent genuine differences in incidence (Table 7.2).
Table 7.2 Organisms causing neonatal meningitis in developing countries.
| Frequency of occurrence | Organisms |
| Common (>10%)a | Klebsiella species E. coli Serratia species Acinetobacter species Salmonella (non-typhoidal) aGBS aS. aureus |
| Uncommon (1–10%) | Pseudomonas species Enterobacter species Other Gram-negative enteric bacilli bS. pneumoniae Enterococci (faecal streptococci) Other streptococci |
| Rare (<;1%) | Coagulase-negative staphylococci S. aureus H. influenzae (usually untypeable) N. meningitidis Anaerobes |
| aReported from some countries but not others. bCan be <;1 month, but more common at age 1–3 months. | |
Gram-negative enteric bacilli, particularly Klebsiella species, cause early- and late-onset meningitis worldwide. Non-typhoidal salmonellae and S. aureus are reported in some countries but not others. S. pneumoniae is more likely to cause post-neonatal infant meningitis but can also cause neonatal meningitis particularly in Africa.3
7.4 Clinical manifestations
The clinical signs in bacterial meningitis are mostly non-specific (Table 7.3).1-7 However, a bulging fontanelle occurs in about 33% of infants with meningitis, seizures in 30% and neck stiffness in 15%. Opisthotonus (Figure 7.2) is a late and sinister sign.
Table 7.3 Clinical signs and estimated frequency in neonatal meningitis.
| Frequency of signs | Clinical signs |
| Common, non-specific (40–60%) | Abnormal temperature (fever or hypothermia) Abnormal behaviour: lethargy or irritability |
| Specific (15–33%) | Bulging fontanelle (33%) Seizures (30%) Neck stiffness (15%) |
| Non-specific (15–40%) | Respiratory symptoms (tachypnoea, distress) Jaundice Vomiting or diarrhoea |
7.5 Diagnosis
The rationale for immediate LP in suspected sepsis is discussed in Chapter 4 (see Section 4.1.3). The common practice of starting empiric antibiotics without performing an LP and relying on the blood culture result to diagnose meningitis is dangerous: 28–38% of neonates with bacterial meningitis have a negative blood culture.13,19 The risk of LP causing respiratory compromise can be reduced by performing the LP with the infant in a sitting or modified lateral position.40
One paper described the interpretation of CSF results as both art and science, noting that in the second century Galen described CSF as a vaporous humour produced in the ventricles that provided energy to the rest of the body and commenting wryly “such theories have not been universally replaced by rationale.”41 The interpretation of neonatal CSF results is complicated by problems defining normal ranges, problems defining meningitis, interpretation of blood-stained CSF and influence of extraneous factors.
One barrier to defining normal ranges is an ethical problem. Although one 1966 South African study performed LPs on well full-term infants in the first day after birth,41 it is not now considered ethical to LP a normal baby. Therefore in deciding that a baby who received an LP, usually for suspected sepsis, is in fact ‘normal,’ it is imperative to exclude viral or other occult infection, which is impossible in practice. Additionally, CSF parameters vary by gestational age and post-natal age.
The problem defining meningitis relates mainly to growth of possible CSF contaminants and situations where the blood culture grows an organism but the CSF does not, with or without changes in CSF microscopy or biochemistry.
7.5.1 Cerebrospinal fluid white cell count
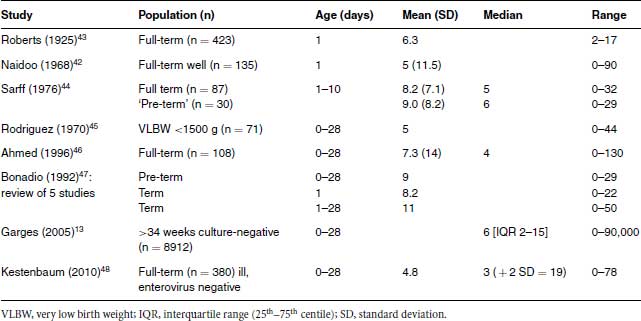
A summary of CSF white cell counts from selected studies13,42-48 is given in Table 7.4. There is quite good concordance between the mean or median white cell counts in the studies, which do not seem to vary greatly with gestation. A US study of infants <;1500 g found no correlation between gestational age and CSF white count in infants who had an LP at birth. However, CSF white count decreased with increasing age.49 This information is not useful to the clinician managing an infant with possible meningitis who wants to know “given the CSF findings, is this infant likely to be infected or can I withhold antibiotics?”
Figure 7.2 Bacterial meningitis causing severe opisthotonus (photo reproduced courtesy of Olivia Swann).
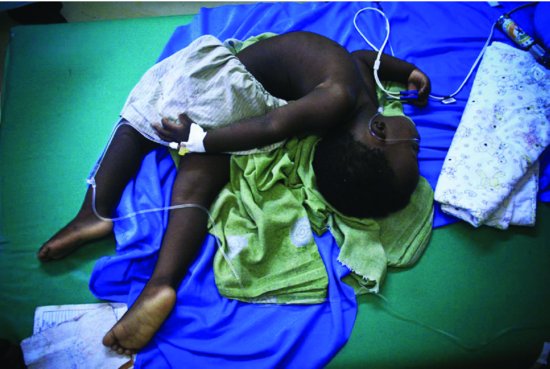
Table 7.4 Normal values for CSF total white cell count (×106/L or per mm3) from selected studies.
The clinician has a number of questions about CSFs.
CSF microscopy often shows large numbers of red cells, either as a result of a traumatic LP or of intraventricular haemorrhage. It is common practice to correct for the presence of red cells in CSF on the basis of the ratio between red and white cells in peripheral blood. This ratio is about 500–1000 to 1 so, if the red cell count is reported as 30 000 say, the clinician ‘allows’ 30–60 white cells as being introduced into the CSF from the blood, not infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


