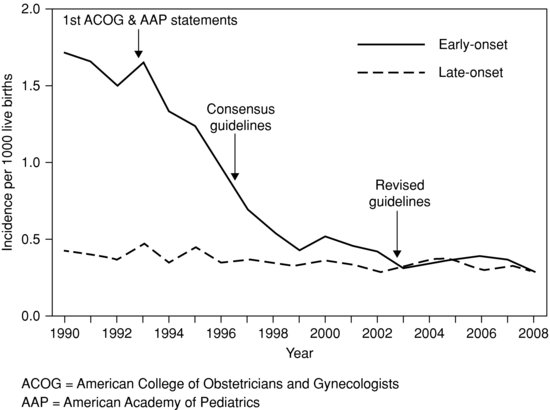
Figure 14.1 Decline in early-onset neonatal group B streptococcal infection in the United States. Reproduced with permission from Reference 17).
IAP for colonized women prevents early-onset neonatal GBS infection,32 which raises two important questions:
Cost and cost-effectiveness is one issue. There is also a need to achieve the optimal balance between the number of women treated with parenteral antibiotics (which medicalizes women and occasionally hazardous) and the number of cases of GBS prevented.
Figure 14.2 Early-onset group B streptococcal infection incidence in Australia and New Zealand, (n = 206, p <; 0.001) 47.
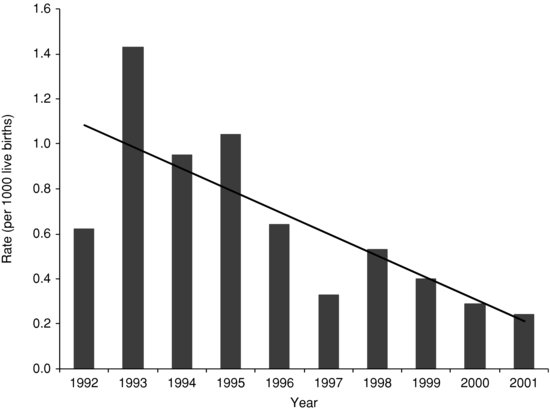
Table 14.2 Risk factors for early-onset neonatal group B streptococcal (GBS) infection.6–8
| Prematurity: risk increases with decreasing gestation and birth weight Spontaneous pre-term onset of labour Spontaneous pre-term rupture of membranes Prolonged rupture of membranes (increases with increasing duration >12 hours) Maternal intrapartum fever >38°C Maternal clinical chorioamnionitis Maternal urinary tract infection with GBS Maternal colonisation with group B streptococcus (GBS) Previous baby with early-onset GBS infection Intrauterine foetal monitoring |
14.1.1.9 IAP and antibiotic resistance
One concern has been that IAP could select for infection with other organisms resistant to penicillin or ampicillin. Most studies have found the incidence of early-onset sepsis due to other organisms has remained unchanged 48–56 or decreased.47,57 Some papers reported an increased incidence of Escherichia coli infections in pre-term infants temporally related to antepartum prophylaxis and some studies reported increasing ampicillin resistance in these E. coli isolates.58–63 Claims that these changes were caused by IAP rather than a chance association ignored the fact that increasing ampicillin resistance was occurring simultaneously elsewhere,64 and that it was biologically implausible that short-term antibiotic prophylaxis would have such a profound effect on resistance.65 Further studies have not suggested a consistent increase in ampicillin-resistant E. coli infections in term or pre-term infants.65–67
14.1.1.10 Management of newborns whose mothers received intrapartum antibiotic prophylaxis
The management of newborns born to mothers who received IAP, particularly mothers known to be GBS-colonized, is problematic. The current IAP recommendation is that women receive penicillin G or ampicillin IV 4 hourly until delivery. A Spanish study showed that the neonatal colonization rate fell from 46% if the baby was delivered less than an hour after antibiotics were started to 2.9% at 2–4 hours and 1.2% after 4 hours.68 While further studies have confirmed that the effectiveness increases after 2 hours,69 the quality of the data on the duration of maternal antibiotics has been questioned.70,71
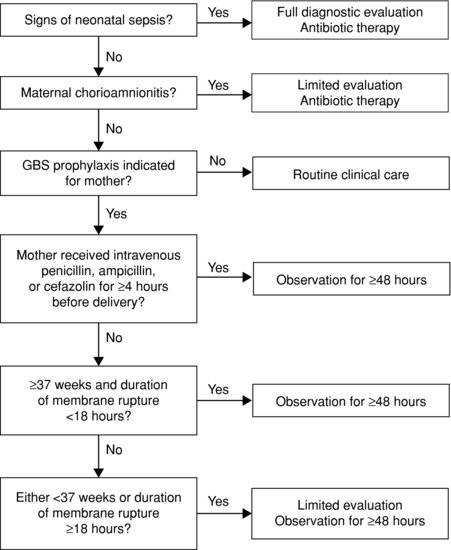
The US CDC recommendations17 on management of all infants, including those born to mothers who received intrapartum antibiotics, are summarized in Figure 14.3. A retrospective Israeli study questioned the need to perform full blood counts and blood cultures on well babies whose GBS-carrier mothers received only one dose of antibiotic before delivery. Full blood counts sent on infants whose mothers received only one dose of intrapartum antibiotics were non-contributory.72 All 11 infants with proven early-onset GBS sepsis were symptomatic. The authors suggest it is reasonable to observe well, full-term babies whose GBS carrier mothers received only one dose of intrapartum antibiotics without performing haematologic investigations, although cautious clinicians will perform a blood culture.72
Figure 14.3 CDC algorithm for secondary prevention of early-onset group B streptococcal (GBS) disease among all newborns (Reproduced with permission from Reference 17).
14.1.2 Coagulase-negative staphylococci
CoNS cause more than half of all late-onset infections in Western neonatal intensive care units, mostly in pre-term infants receiving invasive respiratory support and/or intravenous nutrition.73 CoNS infections can cause true infection but are also frequent contaminants of blood cultures. Contamination can occur at the time of sampling or in the laboratory. Approximately half of all neonatal blood cultures that grow CoNS are thought to be true infections and the rest are contaminants. The best evidence comes from comparisons of clinical findings with quantitative cultures.74–76 However, quantitative cultures are too time-consuming and expensive to perform except as a research tool. Although time to growth of a positive culture correlates usefully with quantitative cultures,74–76 all studies of neonatal CoNS infection need to be read with caution because a significant proportion of reported cases may not be truly infected. In particular, reports of CoNS causing early-onset infection on the first day of life and of causing late-onset meningitis in the absence of predisposing factors (e.g. shunt, surgery) should be treated with scepticism. Of course the epidemiologist can afford a great deal more scepticism than the clinician. Nevertheless, many infants treated with antibiotics for positive blood or CSF CoNS cultures are probably not infected.
14.1.2.1 Epidemiology of coagulase-negative staphylococcal infections
The definition of CoNS sepsis varies and can greatly affect reported incidence. A definition based on positive cultures plus clinical features compatible with sepsis is not very effective at excluding contaminants, because possible sepsis is the usual indication for blood culture. In the United States it is common to take two blood cultures at the time of investigation for possible sepsis and many studies specify that both have to be classified epidemiologically as a true case. In other countries, the definition of CoNS sepsis may require one or more abnormal laboratory tests (e.g. CRP, I:T ratio).
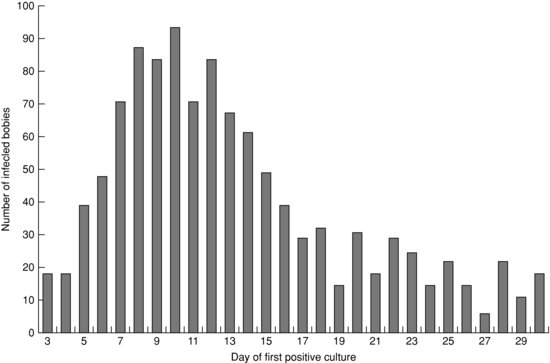
A prospective multi-centre NICU-based study in Australia and New Zealand defined CoNS sepsis as a pure growth of CoNS from blood or CSF plus clinical sepsis plus at least one abnormal haematologic test (I:T ratio, white count or platelet count) or raised serum C-reactive protein.73 There were 1281 cases of CoNS sepsis in 10 years, comprising 57.1% of all late-onset infections. The incidence of CoNS sepsis was 3.5 episodes per 1000 live births. Most infected babies (71%) were 24–29 weeks of gestation at birth (mode 26 weeks). Half of all babies’ positive CoNS culture was in week 2 (mode 10 days, see Figure 14.4). Five cases of meningitis were reported (incidence 0.4% of all CoNS infections). Twenty nine babies (2.3%) had CoNS septicaemia in association with necrotizing enterocolitis. Four babies (0.3%) were assessed as having died from CoNS infection, while CoNS infection was assessed as possibly contributing to the death of an additional 20 babies (1.6%). The mortality of 0.3% directly attributed to CoNS infection was significantly lower than the 13.1% from Staphylococcus aureus (relative risk (RR) = 36.1 (95% CI 13–100.2) or 14.2% from Gram-negative bacilli (RR = 45.5, 95% CI 16.8–123.3) in the same cohort.73
Figure 14.4 Day of first positive culture of coagulase-negative staphylococci from blood or CSF. Infants <;3 days old are not included. (Reproduced with permission from Reference 72).
The incidence of CoNS rises with falling birth weight, as for all late-onset neonatal infections, due to both immaturity and increased length of stay which are major independent risk factors.77 In one study, the rate of CoNS infection was 44.5 times higher in infants <;750 g than in infants >2000 g, but only 5.3 times higher after allowing for length of stay.77 Risk factors for CoNS include prematurity, intravascular catheters particularly intracardiac catheters and endotracheal intubation. Parenteral nutrition fluids, particularly lipids, are a rich growth medium and are a risk factor for CoNS sepsis in addition to the presence of an intravascular catheter.78 CoNS are the most frequent cause of central line-associated bacteraemias (CLABs).75,79
14.1.2.2 Microbiology of coagulase-negative staphylococci
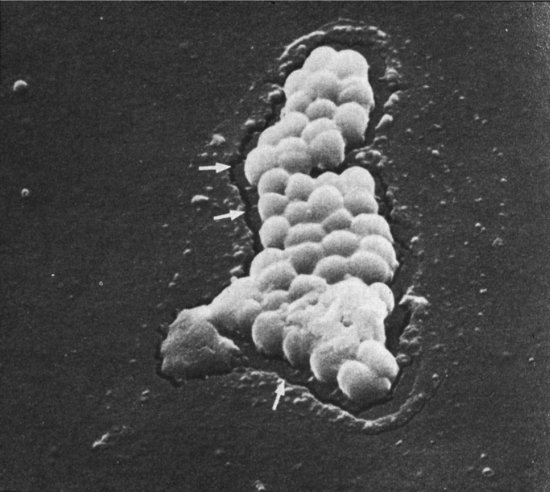
There are 31 species of CoNS, of which 13 colonize human skin and 7 cause neonatal infections: S. epidermidis, S. haemolyticus, S. hominis, S. warneri, S. saprophyticus, S. capitis and S. cohnii. S. epidermidis are responsible for 50–80% of colonization and 60–93% of CoNS bloodstream infection.16 Capsular polysaccharide adhesins help CoNS stick to skin, mucosal surfaces or indwelling intravascular catheters or intracerebral shunts. Some CoNS produce an exopolysaccharide ‘slime’ layer mainly made of N-acetylglucosamine, which acts as a biofilm and protects the organism from host defences and from antibiotics. Slime production has been linked to virulence and also to persistent CoNS infection.78 Interestingly, there is in vitro evidence that the slime produced by S. epidermidis can inhibit the penetration of fluconazole into mixed Candida and S. epidermidis biofilms while Candida albicans biofilm protects CoNS from vancomycin,80 which could explain mixed neonatal infections with Candida and S. epidermidis. CoNS adhere, but also secrete enzymes that allow colonies to burrow into the surface of silastic catheters (Figure 14.5).
Figure 14.5 Scanning electron micrograph showing coagulase-negative staphylococci eroding into the surface of a silastic catheter, with some biofilm at the bottom of the colony.
Staphylococcus lugdunensis is more pathogenic than most CoNS strains, sometimes approaching S. aureus in virulence. It can cause endocarditis in older children and adults, although not reported in neonates yet. Like other CoNS it is often an asymptomatic commensal that has been reported to cause catheter-associated bacteraemia. Some laboratories report S. lugdunensis isolates. Clinicians should only be concerned if they suspect sepsis but should not be more worried about colonization with S. lugdunensis than they would by S. aureus colonization.
CoNS can rarely be acquired from the maternal genital tract at birth, but most infants become colonized soon after birth with other strains. Nosocomial CoNS colonization is commonly via the hands of staff.16
14.1.2.3 Clinical features of coagulase-negative staphylococcal infection
There are no specific clinical features of CoNS infection, which is usually indolent, presenting with apnea, bradycardia, increased ventilatory requirements, irritability and feeding intolerance. Fever may or may not be present.81,82 In a retrospective study, fulminant sepsis, defined as death within 48 hours of a positive blood culture, occurred in 4 of 277 infants >3 days old who grew CoNS in their blood culture.83
14.1.2.4 Early-onset coagulase-negative staphylococcal infection
A retrospective study identified 11 infants out of a population of approximately 7800 extremely low birth-weight infants <;1500 g who were thought on chart review to have true early-onset CoNS sepsis (10 on day 1, 1 on day 2) in association with ventilator-dependent respiratory distress. Three had repeat positive cultures, 4 had pulmonary haemorrhage and 3 died.84 The retrospective nature of this study, the potential that an extremely pre-term infant would have a similarly severe course without sepsis and the lack of autopsy data makes it debatable whether these infants had true early-onset CoNS infection.
14.1.2.5 Laboratory findings in coagulase-negative staphylococcal infection
There are no specific laboratory features of CoNS infection. Thrombocytopenia occurs in 15–25% of infants with acute CoNS infection, but this does not distinguish CoNS from other bacterial and fungal causes of sepsis.85,86
14.1.2.6 Management of coagulase-negative staphylococcal infection
Most CoNS strains carry the mecA gene and have in vitro resistance to cloxacillin, so vancomycin is generally considered the antibiotic of choice for treating CoNS infection until antibiotic sensitivities are back. However, because of concerns about the selection of vancomycin-resistant enterococci, it is common to use a semi-synthetic penicillin (oxacillin, cloxacillin, flucloxacillin, dicloxacillin) plus an aminoglycoside, usually gentamicin, for empiric treatment of suspected late-onset sepsis (see Section 5.1.2). There are no RCTs, but sequential studies comparing empiric vancomycin plus aminoglycoside with empiric oxacillin/cloxacillin plus aminoglycoside for suspected late-onset sepsis showed no difference in outcomes from CoNS infection treated with either regimen.83,87,88 While it is generally recommended to change to vancomycin if CoNS infection with a resistant strain is proven, infants with oxacillin-resistant organisms have often responded to the oxacillin plus aminoglycoside regimen by the time cultures are back.83,87–89 This may be because aminoglycosides have some activity against CoNS, it may be due to catheter removal, or because the isolates were contaminants. A Dutch study of 163 infants with CoNS bacteraemia reported that 140 had a cefazolin-susceptible strain (86%). The authors reported a good response to cefazolin in most infants with cefazolin-sensitive or cefazolin-resistant strains, although 22% of infants failed cefazolin and the response was better in those whose central venous catheter was removed.89
The need for removal of central lines in CoNS bacteraemia is considered in Section 6.10.1. Early central venous catheter removal improves the likelihood of resolution of CoNS but clinicians often want to preserve a precious catheter. About half of all infants can be successfully treated without removing the central line.79,90 However, bacteraemia which persists >4 days does not resolve without central venous catheter removal.90
The optimal duration of antibiotics for CoNS bacteraemia is unknown. A Dutch group hypothesized that most CoNS infections respond within 3 days and stopped antibiotics after 3 days if infants with CoNS infection had responded clinically, had normal platelets and had no catheter in situ (never present or removed). All 80 infants treated with 3 days of antibiotics recovered without relapse.91
14.1.2.7 Persistent coagulase-negative staphylococcal infections
CoNS infections have a propensity to persist, with or without fever or other signs of infection, and often in association with thrombocytopenia.92,93 Endocarditis should always be considered in any child with persistent bacteraemia. In one study, 5 of 58 infants with persistent CoNS bacteraemia had right-sided endocarditis in association with umbilical catheters in the right atrium.92 Most cases of persistent CoNS bacteraemia are thought to be due to endovasculitis, to which central venous catheters probably predispose, although persistent CoNS bacteraemia can occur in infants who never had a central venous catheter.
In one study, 31 (18%) of 171 infants with CoNS bacteraemia had persistent infection. The incidence of thrombocytopenia was 84% for persistent CoNS infection compared with 13% for those whose infection resolved.93
In a retrospective case-control study, 52 infants with persistent CoNS bacteraemia >48 hours were significantly more likely than controls to have feed intolerance and to need ventilatory support, inotropes, and blood transfusion but mortality was not increased. Risk factors for persistent infection were duration of parenteral nutrition, hydrocortisone, antibiotics, and mechanical ventilation prior to infection.94 In another study, endotracheal intubation, central venous catheters and biofilm production were significant risk factors for persistent infection.95 An Israeli study found persistent CoNS bacteraemia resolved quicker in breastfed infants.96
A retrospective Dutch study identified 137 infants with CoNS bacteraemia, which persisted in 18 (13%) who had three positive cultures at least 48 hours apart and were treated with vancomycin and rifampicin (rifampin). The authors reported a rapid response to rifampicin, although the retrospective design casts doubt on the validity of this observation.97
14.1.2.8 Outcome of coagulase-negative staphylococcal infections
The mortality of CoNS infection is low, 0.3% in the largest prospective study73 and 1.4% in a retrospective US study.83 A very large multi-centre study identified 16 629 infants with 17 624 episodes of CoNS infection in 248 NICUs, and classified the CoNS infections as definite (10%), probable (17%) and possible (73%). Surprisingly, the mortality was significantly lower in infants with CoNS infection than in controls matched for gestational, birth weight and Apgar score for all three categories.98 It does not seem credible that CoNS infection would prevent deaths, and the retrospective nature of the study makes it likely that the results are confounded.
Other studies have shown that CoNS bacteraemia is associated with prolonged duration of NICU stay, on average 14 days longer than controls, and with significantly increased hospital costs.99 A follow-up study of over 6000 infants born weighing 401–1000 g assessed at 18–22 months found that infants with CoNS infection were significantly more likely than non-infected infants to have impaired neurodevelopment.100
14.1.2.9 Prevention of coagulase-negative staphylococcal infections
Evidence from before and after studies suggests that improved hand hygiene101–103 and clusters of infection control measures involving care of peripheral and central lines104 reduce the incidence of CoNS infections.105 While these measures are probably truly effective, our previous caveat about distinguishing true infections from contaminants is pertinent. The measures might reduce blood culture contaminants rather than true CoNS infections. In one study improved hand hygiene was associated with a significant reduction in CoNS blood culture contaminants, whereas the lesser reduction in ‘true’ CoNS infections did not reach statistical significance.106
There is RCT evidence that early introduction of enteral nutrition can prevent CoNS infections. Infants <;1750 g randomized to trophic feeds of 0.5–1 mL/hour plus parenteral nutrition from day 3 until ventilator support finished had significantly fewer infections than those on parenteral nutrition (mean 0.5 vs 1.2 episodes of culture-proven sepsis, mainly CoNS).107 A retrospective Canadian study found that achieving full enteral feeds was associated with an 85% reduction in CoNS infections.108
14.1.3 Staphylococcus aureus
S. aureus was a major cause of hospital-acquired neonatal sepsis between the 1950s and the 1970s, causing major nursery outbreaks. These were often associated with skin sepsis and omphalitis (see Chapter 13), while osteomyelitis and septic arthritis (see Chapter 9) were rare, serious complications. S. aureus strains produce an enzyme, coagulase, which is detected in the laboratory to distinguish them from CoNS. S. aureus has a cell wall with two major components, peptidoglycan and teichoic acid, and an outer capsule. Antibiotic resistance genes are mainly carried on mobile genetic elements which include a so-called resistance island. The organism can also carry gene clusters called pathogenicity islands coding for production of exotoxins like toxic shock syndrome toxin and for enterotoxins. The basis for methicillin resistance of all MRSA isolates is the mecA gene, which codes for a penicillin-binding protein with greatly reduced affinity for β-lactam antibiotics. The Panton–Valentine leukocidin (PVL) gene, which is associated with increased virulence, can be carried by strains of MRSA and strains of methicillin-sensitive S. aureus (MSSA).109
S. aureus can cause both early- and late-onset infections. In Western countries, early-onset S. aureus infections are rare,110,111 and most empiric antibiotic regimens for early-onset sepsis do not include an anti-staphylococcal antibiotic (see Chapter 5). However, the mortality of early-onset S. aureus infection is high: in one study the incidence of early-onset S. aureus infection (all MSSA) was 0.08 per 1000 live births but the mortality was 39%.110 Empiric antibiotic regimens for possible early-onset infection may need to be reviewed if MSSA or community MRSA strains emerge as a common cause of early-onset infection,112 although currently MRSA are much more likely to cause late-onset infections.109–113 In most developing countries, in contrast, MSSA is an important early-onset pathogen.114 S. aureus is also an important late-onset pathogen worldwide (see Chapter 2) and it is recommended that all empiric regimens for suspected late-onset sepsis should provide anti-staphylococcal cover (see Chapter 5).
The clinical presentation of S. aureus infections will mainly be considered under the relevant chapters. S. aureus can cause skin and soft tissue infections including omphalitis, mastitis, staphylococcal scalded skin syndrome and toxic shock syndrome (see Chapter 13), osteomyelitis and septic arthritis (see Chapter 9), pneumonia (see Chapter 8), endocarditis and bacteraemia, including CLAB. S. aureus can cause central nervous system shunt infections and are a rare cause of meningitis in the absence of CNS shunts. S. aureus meningitis can occur secondary to endocarditis, due to rupture of a mycotic aneurysm, secondary to epidural abscess and rarely in isolation secondary to bacteraemia.109,110 Similar to CoNS bacteraemia, although S. aureus meningitis is rare, it is recommended to perform an LP for an infant with S. aureus bacteraemia who did not have an original LP because of the danger of under-treatment if meningitis is missed.
14.1.3.1 Treatment of infections due to Staphylococcus aureus
S. aureus were originally universally susceptible to penicillin, but strains soon emerged that produced β-lactamases (called penicillinases). About 10% of strains of S. aureus are sensitive to penicillin and have a much lower MIC for penicillin than for semi-synthetic penicillins such as oxacillin. Penicillin G is the antibiotic of choice for invasive infections due to penicillin-sensitive strains of S. aureus.
For methicillin-sensitive penicillin-resistant strains, the treatment of choice is a β-lactamase-resistant semi-synthetic penicillin, e.g. cloxacillin, flucloxacillin, oxacillin or nafcillin. There is no good evidence for adding an aminoglycoside or rifampin (rifampicin) for synergy. First generation cephalosporins such as cefazolin or cephalothin are also active against MSSA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


