Fig. 4.1
Screening in a social ecological context
Timely screening for multifaceted family psychosocial risk is a means by which treatment needs and follow-up care for the patient and family can be identified in an effective and inclusive manner to facilitate efficient assessment and delivery of evidence-based care matched to patient and family need. In light of a recent comprehensive review of the literature on screening in pediatric cancer (Kazak et al. 2012), this chapter includes an updated search, completed in October 2014, to identify recent papers on psychosocial screening in pediatric cancer. We used the same keywords as the earlier review (“pediatric oncology” or “pediatric cancer” or “childhood cancer”) AND (screen* or tool* or assess* or classify* or categorize or evaluate or “psychosocial risk” or “psychosocial need” or “psychosocial care” or at risk” or “level of risk” or “identify risk” or distress or parents) AND (NOT survivor*) and databases (PsycInfo, Cinahl, PubMed, and Health and Psychosocial Instruments Database). In addition, authors of recent papers were contacted to obtain copies of relevant presentations and prepublication work.
Models for Standardized Screening
Three primary models of risk screening were identified in a previous review (Kazak et al. 2012) – 1) the Pediatric Preventative Psychosocial Health Model (PPPHM), 2) the Family APGAR (Adaptability, Partnership, Growth, Affection, and Resolve) approach, and 3) the HEADSS (Home, Education, Activities, Drugs, Sexuality, and Suicide/Depression) framework. No additional models of psychosocial risk screening in pediatric cancer were found in the literature between 2011 and 2014.
The Pediatric Psychosocial Preventative Health Model (PPPHM; Fig. 4.2) is based on a public health framework and used to conceptualize families with varying levels of psychosocial risk along with interventions matched to risk (Kazak 2006). Each of the PPPHM levels is described below followed by a case example.
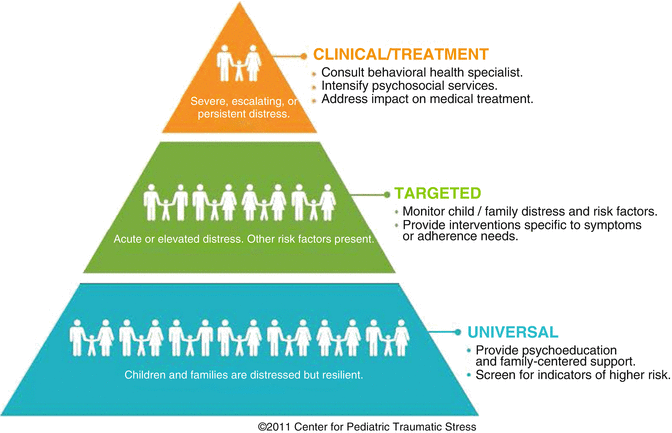

Fig. 4.2
Pediatric Psychosocial Preventative Health Model (PPPHM) (Reproduced with permission from the Center for Pediatric Traumatic Stress (CPTS) at Nemours Children’s Health System © 2011. All rights reserved. The PPPHM may not be reproduced in any form for any purpose without the express written permission of CPTS. To obtain permission to use reproduce the PPPHM, contact Anne Kazak, PhD ABPP at anne.kazak@nemours.org)
At the base of the pyramid are Universal families, who are understandably concerned or distressed about their child’s health problem but who are generally resilient and able to cope and adapt to their child’s illness and treatment.
Universal Level Case Example: Max is a 15-year-old recently diagnosed with resilient leukemia. His parents are married. They work full time outside the home but expect that their employers will afford them some flexibility over the next few months. Max has two siblings, ages 12 and 17, and a large family support system available for help. Max’s parents consider him a bit of a worrier but otherwise a popular student who learns easily. His mother reports a history of frequent migraine headaches and appears sad and tired at clinic visits.
The middle tier consists of Targeted families, with preexisting concerns or difficulties that may contribute to continuing or escalating vulnerability during treatment.
Targeted Level Case Example: Aiden is a 9-year-old recently diagnosed with a rhabdomyosarcoma. His teachers have noted difficulties in attention and some challenges in learning last year and this year. His parents are executives in local businesses who separated last year and have occasional disputes about custody and child support. Both parents are present at clinic visits.
At the tip of the pyramid are Clinical families, with one or more preexisting, chronic, and complex problems and resulting greatest need for prompt and often intensive intervention. Clinical Level Case Example: Sophia is a 5-year-old recently diagnosed with a medulloblastoma. She has three younger siblings, ages 3 and 2 years and 8 months. Her parents have many financial worries (e.g., concerns about paying phone and utility bills, rent, etc.), few people to assist them, and general apprehensiveness about treatment and its impact on their daughter and family. Sophia’s parents indicate that she has several behavioral and developmental concerns (e.g., moodiness, anxiety, problems in kindergarten) and, on screening, endorses a number of behavioral concerns for at least one of the siblings. In addition, her mother has a history of anxiety and endorsed symptoms of acute stress since her daughter’s diagnosis.
Using the PPPHM as a guide, treatment options vary by level. Many of the services currently provided in pediatric settings (e.g., social workers, child life specialists, chaplains, creative arts programs, family-centered care programs, financial counselors, etc.) provide a broad undergirding of care that will address many of the needs of Universal families. There are many evidence-based interventions developed that are appropriate for families at the Targeted level. These include cognitive behavioral therapy for pain and behavioral or multicomponent interventions for adherence to medical regimens (see http://www.apadivisions.org/division-54/evidence-based/). At the Clinical level, behavioral medicine teams are usually necessary to assess and provide generally more intensive interventions in addition to Universal services that families would receive. The PPPHM provides a “snapshot” of the family’s risks and resilience. Screening always necessitates clinical follow-up assessment to determine a treatment plan. Continued monitoring of risk for all families is critical to capture changes in risk over time. The literature review identified eight papers that referred to the PPPHM as a model or guide for pediatric populations.
The Family APGAR (Adaptability, Partnership, Growth, Affection, and Resolve) provides quantitative data on individual family member’s satisfaction with their family’s functioning based on the APGAR components. The items are intended to measure individual family member’s perception of family functioning. The Family APGAR also allows for the integration of physician knowledge of the family and follow-up discussions with the family member to gain qualitative data of the individual family member’s satisfaction and family functioning based on the quantitative APGAR data (Smilkstein 1978).
The HEADSS framework (Home, Education, Activities, Drugs, Sexuality, and Suicide/Depression) interview guide aims to engage adolescents and young adults (AYAs) in preventative healthcare throughout the course of their cancer treatment (Yeo and Sawyer 2009). Providing physicians with a developmentally appropriate guide to engage adolescents and young adults in queries of specific areas of psychosocial risk is important in building rapport and ensuring patient-centered care. Although there is limited empirical basis for the HEADSS interview, the content is consistent with general adolescent preventative care (Goldenring and Rosen 2004).
Screening Methods and Evidence-Based Tools
There are potentially many different means of conducting screening. Before discussing specific approaches and measures, the results of a listserv posting conducted as part of the Psychosocial Standards of Care Project for Childhood Cancer (PSCPCC) are noted (Kupst MJ, 2015, Personal Communication). Queries were posted in July 2013 to listservs of the Society of Pediatric Psychology (SPP) and the International Society of Pediatric Oncology (SIOP) asking what measures were used in clinical care for screening and assessment of children, adolescents, and young adults with cancer. Although not a scientific survey, the results indicated that the most frequently used screeners were the Psychosocial Assessment Tool (PAT; Pai et al. 2008), the Strengths and Difficulties Questionnaire (SDQ; Goodman 2001), the Child Behavior Checklist (CBCL; ASEBA 2015), the Distress Thermometer (DT; National Comprehensive Cancer Network® (NCCN®) 2003), the Behavior Assessment System for Children (BASC/BASC-2; Reynolds and Kamphaus 2004), and the Brief Symptom Inventory (BSI; Derogatis 2000).
Methods for Screening
One method of risk assessment is to use a battery of validated measures (e.g., well-known measures of depression, anxiety, child behavior).1 The advantage of these approaches is the use of established psychometrically strong instruments, usually specific to a construct (e.g., quality of life, behavior, depression) and often with clinical cutoff scores. The disadvantages are that multiple measures are often necessary to assess relevant outcomes. The number of items on these batteries can be significant and the participant burden and administration time can become longer than is feasible in a medical setting. Scoring and interpretation may also necessitate a mental health professional; adding another step can slow the communication of results and may be problematic in some settings without such staff. One of the more creative applications of this approach, measuring quality of life in the Netherlands, is KLIK, a Dutch acronym roughly translated in English as Mapping Quality of Life in Clinical Practice, which provides a patient and family ePROfile developed from generic and illness-related questionnaires. KLIK provides the healthcare team with direct access to patient responses, which increases communication and provider satisfaction in care (Haverman et al. 2014).
A second approach is the very brief screeners, exemplified by the Distress Thermometer (DT) and discussed below.
Another method is structured clinical interviews. Such interviews typically include standardized questions about the nature, severity, and duration of symptoms, often with the goal of determining a diagnosis. Exemplified by the HEADSS, discussed above (e.g., HEADSS 3.0; Goldenring and Rosen 2004), structured clinical interviews assess a broad range of topics in detail and facilitate rapport. Although they provide clinicians with guidance regarding which questions to ask and how to ask them, they tend to be susceptible to interviewer drift and social desirability. The standardized structured clinical interview such as HEADSS also requires trained staff and increases the burden on clinical staff, and the administration time can be problematic in healthcare settings. The structured clinical interview differs from a clinical assessment in which the clinician is asking questions about particular areas of interest but using a less formally structured protocol to do so.
Yet another approach is the use of single relatively short standardized instruments. Most focus solely on child behavior (e.g., the Pediatric Symptom Checklist) or parenting stress (e.g., the Parenting Stress Index). The Beck Youth Inventory II was found to be a feasible approach for screening depression and anxiety in adolescents in oncology treatment (Kersun et al. 2009). SCREEM-RES (Social, Cultural, Religious, Economic, Education, and Medical) Family Resource Survey questionnaire was developed to identify areas that families need support in order to increase the family’s capacity to cope with the child’s cancer (Panganiban-Corales and Medina 2011).
The Psychosocial Assessment Tool (PAT) is the only measure developed specifically for pediatric oncology, guided by research evidence and clinical experience to assess (parental report) a range of potential risks across the family’s social ecology. The PAT is discussed below in more detail.
Two Empirically Supported Tools
The Distress Thermometer (DT; Fig. 4.3; NCCN® 2014) uses a graphic representation of a thermometer, generating a 1–10 unidimensional rating of how distressed the respondent has felt in the past week. The DT may also be used with a problem list of practical problems (e.g., housing, insurance), family problems (e.g., dealing with partner, children), emotional problems (e.g.., worry, sadness), spiritual and religious concerns (e.g., relating to God, loss of faith), and physical problems (e.g., pain, nausea) (NCCN 2003). The DT has been used quite extensively in adult oncology and more recently in pediatric cancer (Patel et al. 2011). Data from Patel et al. (2011) were extracted for patients and their mothers at the end of life, indicating that the DT was helpful in tracking changes at this point in treatment (Patel et al. 2011). The DT is feasible in screening for distress in children with cancer or other chronic illness and correlated with both child and parent measures of depression, anxiety, pain, and fatigue (Zadeh et al. 2014). The Distress Thermometer for Parents (DT-P) was also developed and validated for parents of children with chronic illnesses, showing associations of its 10-point Likert scale with parental distress (Haverman et al. 2013).
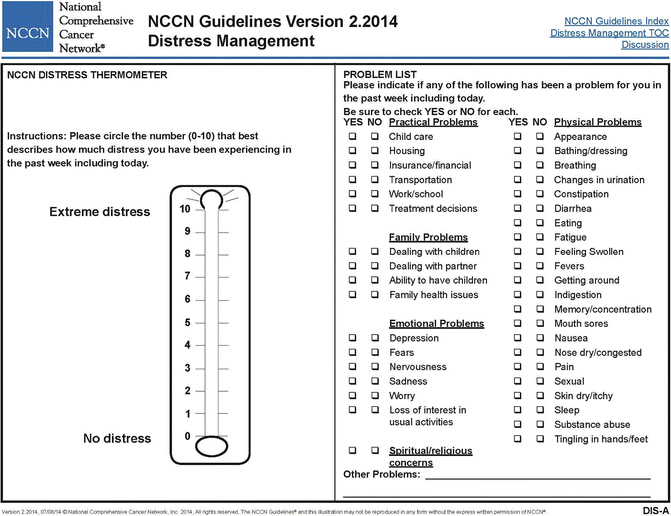

Fig. 4.3
NCCN® Distress Thermometer (Reproduced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management V.2.2014. © 2014 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.)
The advantages of the DT are brevity, simplicity, and focus on the self-reported distress of the respondent. Its simplicity allows for it to be completed by children and parents. However, a single score of one dimension (distress) may provide limited clinical information and is not highly specific in providing direction for needed intervention. The use of problem lists on the DT contributes information about areas of concern, although it adds slightly to the length, which is usually 3–5 minutes.
The Psychosocial Assessment Tool (PAT; Fig. 4.4; Pai et al. 2008), a brief (5–10 minutes administration time) screener of family psychosocial risk based on the PPPHM’s trilevel of risk classification, was developed for families of children with cancer. In addition to the total score, there are seven subscales (structure/resources, family problems, social support, stress reactions, family beliefs, child problems, and sibling problems). The psychometric properties of the PAT are strong (Pai et al. 2008) and PPPHM risk classification is generally stable across 4 months (Alderfer et al. 2009). Detailed information about the history, use, and research on the PAT is detailed in a recent paper (Kazak et al. 2015). The all-literacy English and Spanish versions of the PAT (4th-grade reading level) are being tested in a current multisite study (ACS RSG-13-015).
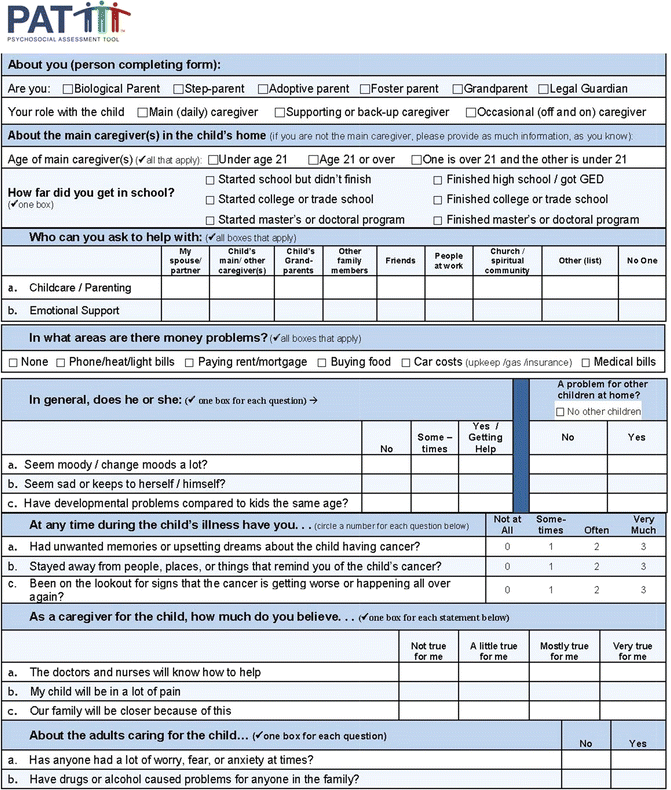

Fig. 4.4
Sample items from the Psychosocial Assessment Tool (PAT) (Reproduced with permission from the Center for Pediatric Traumatic Stress (CPTS) at Nemours Children’s Health System © 2014–2015. All rights reserved. The PAT image above is comprised of sample items from each of the PAT subscales. The PAT image may not be reproduced in any form for any purpose without the express written permission of CPTS. To obtain permission to use or view the most recent version of the PAT, please contact CPTS at psychosocialassessmenttool@nemours.org)
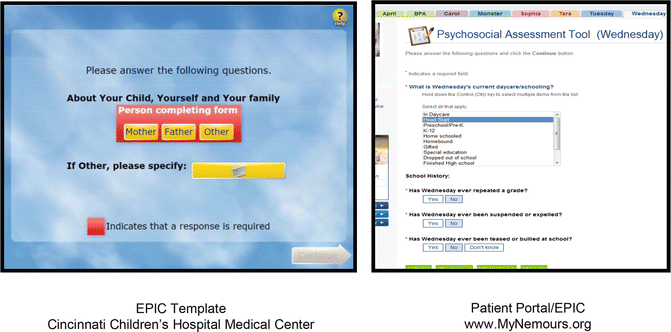
Recent papers support the use of a Canadian adaptation of the PAT (Barrera et al. 2014), associations between socioeconomic variables and overall risk level over a 1-year period (Karlson et al. 2013), and feasibility in survivorship care (Gilleland et al. 2013). PAT can be administered in paper and pencil, via REDCap on a tablet computer, or using a web-based version. Interfaces with electronic health records (EHRs), specifically (EPIC; www.epic.com) at Nemours Children’s Health System and Cincinnati Children’s Hospital Medical Center, and in the patient/family portal at Nemours (Fig. 4.5), are available as well.