FIGURE 10.1 Normal intracranial vasculature. Coronal (left panel) and axial (right panel) magnetic resonance angiograms (MRAs) demonstrating normal intracranial vasculature. (a) internal carotid artery; (b) middle cerebral artery; (c) anterior cerebral artery; (d) anterior communicating artery (arrowhead, left panel only); (e) vertebral artery; (f) basilar artery; (g) posterior cerebral artery; (h) posterior communicating artery (arrow, right panel only).
■ PATHOPHYSIOLOGY OF AIS
There are multiple potential mechanisms that lead to AIS, and there are multiple steps in the chemical cascade during an acute AIS that lead to eventual death of brain tissue. When an artery becomes occluded by a thrombus in the vessel wall or by an embolus from a proximal source, blood flow is cut off to the region supplied by that artery. Alternately, during systemic hypoperfusion from such causes as sepsis or cardiogenic shock or in the case of severe distal carotid stenosis (moyamoya or sickle cell-associated vasculopathy), the border zone between different vascular territories receives less blood flow and can cause “watershed infarcts.” Either by thrombus, embolus, or hypoperfusion, the final common pathway is a complex chain of cellular events. Lack of oxygen and glucose leads to mitochondrial dysfunction, ion pump failure, calcium influx, and glutamate-induced neurotoxicity. Whether cell necrosis or apoptosis occurs depends on a number of factors, including the amount of collateral blood flow available to the injured brain tissue, the oxygen demand in the tissue itself, and the time it takes to restore blood flow to the injured tissue. The time that it takes to restore or improve blood flow to the injured tissue, “time to reperfusion,” is a factor that intervention strategies aim to shorten in both pediatric and adult AIS. During a stroke, a core of infarcted tissue in which cells have undergone apoptosis and/or necrosis occurs and is often surrounded by an area that is still salvageable known as the “ischemic penumbra.” Without improvement of blood flow into the tissue, irreversible cell death occurs. In theory, a TIA occurs when blood flow in an artery is restored before permanent tissue infarction occurs.
■ CLINICAL PRESENTATIONS AND DIFFERENTIAL DIAGNOSIS
Pediatric AIS is underrecognized within the medical community, despite the considerable and costly long-term disability and residual behavioral, cognitive, emotional, and functional deficits. In one study, the median time to diagnosis of pediatric AIS was over 24 hours (8). Part of the reason for this delay in diagnosis of childhood AIS may be a low index of suspicion by health care professionals. Additionally, the presenting symptoms in children with AIS are more heterogeneous than in adults. Children are much more likely than adults to present with headaches, seizures, or altered mental status as the first sign of stroke. Younger children in particular are more likely than older children to present with an encephalopathy or a decreased level of consciousness; however, a detailed neurologic examination may actually uncover a subtle, new focal deficit. In the study mentioned above, although 86% of children with confirmed AIS had a focal neurologic deficit when first seen by a physician, the presence of a focal deficit was not associated with a more rapid time to stroke confirmation (8).
Hemiparesis is the most common presenting sign of acute AIS in children, and the MCA is the most commonly affected vessel (2,5). Children may also present with focal cortical signs such as aphasia, visual field cuts, or neglect, but deficits depend on both the location of the stroke and the age and developmental stage of the child. For instance, AIS in the posterior circulation can present with ataxia, vertigo, emesis, or cranial nerve palsies. Unfortunately, this symptom complex is often mistaken by families and physicians for a viral infection (8).
If a motor deficit occurs, it is often most pronounced upon presentation but sometimes may exacerbate over minutes or hours. Deficits may even fluctuate with resolution to normal and then back to paresis or plegia. AIS in children with watershed distribution infarction may have weakness that is more proximal than distal.
The differential diagnosis for AIS is broad and includes intracerebral hemorrhage, cerebral venous sinus thrombosis, posterior reversible encephalopathy syndrome, complicated migraine (with or without concurrent headache), seizure with a postictal Todd’s paralysis, brain tumor, infections like meningitis, abscess, and encephalitis, and demyelinating diseases like multiple sclerosis, acute disseminated encephalomyelitis, or neurodegenerative diseases, metabolic or mitochondrial diseases, metabolic derangements like hypoglycemia or intoxication, and conversion disorder. In one prospective cohort, 21% of children initially thought to have AIS had stroke mimics (9). Questions about family history of migraines or epilepsy may reveal important clues for the correct diagnosis. Timely triage and neuroimaging studies help to eliminate these alternate diagnoses so that AIS diagnosis and treatment are not delayed.
■ ACUTE DIAGNOSTIC WORKUP AND MANAGEMENT
The emergency room evaluation of a child with suspected AIS should center on diagnosis verification by history and physical examination, vital sign review, laboratory studies, and neuroimaging. The time at which the child was last seen normal should be a crucial part of the history since treatment may vary depending upon the acuity of the stroke. A thorough neurologic examination should be performed, and the Pediatric NIH Stroke Scale score, a measure that has excellent interrater reliability and predictive validity for outcome, should be performed immediately to assess clinical stroke severity (10,11). Acute studies should include electrocardiography, complete blood count with differential, electrolytes, blood urea nitrogen, creatinine, blood glucose, prothrombin time, international normalized ratio, partial thromboplastin time, and toxicology screen. These essential, initial screening tests may rule out stroke mimics and sometimes, as in the case of severe hypoglycemia, reverse the stroke-like symptoms with correction of the underlying problem. As soon as the child is stable, urgent neuroimaging should be performed. A noncontrast HCT should usually be the first image performed since it is fast, does not require sedation in most cases, and is widely available. A HCT is sensitive for acute hemorrhage, and can also be used to rule out large mass lesions. In AIS, noncontrast HCT may be normal early after symptom onset, but occasionally subtle findings of infarction may appear. These include the “hyperdense artery sign” which represents acute clot in an artery (Figure 10.2A), obscuration of the putamen, or diminished differentiation of the gray-white matter junction. In one study, an AIS was not detected in 62 of 74 children who had a noncontrast HCT as their first neuroimage. In another study, the stroke was not detected in 47% of acute HCTs (4). However, a normal HCT is still helpful since it excludes hemorrhage or tumor, and acute treatment for presumed AIS can continue while more definitive imaging with MRI is arranged.
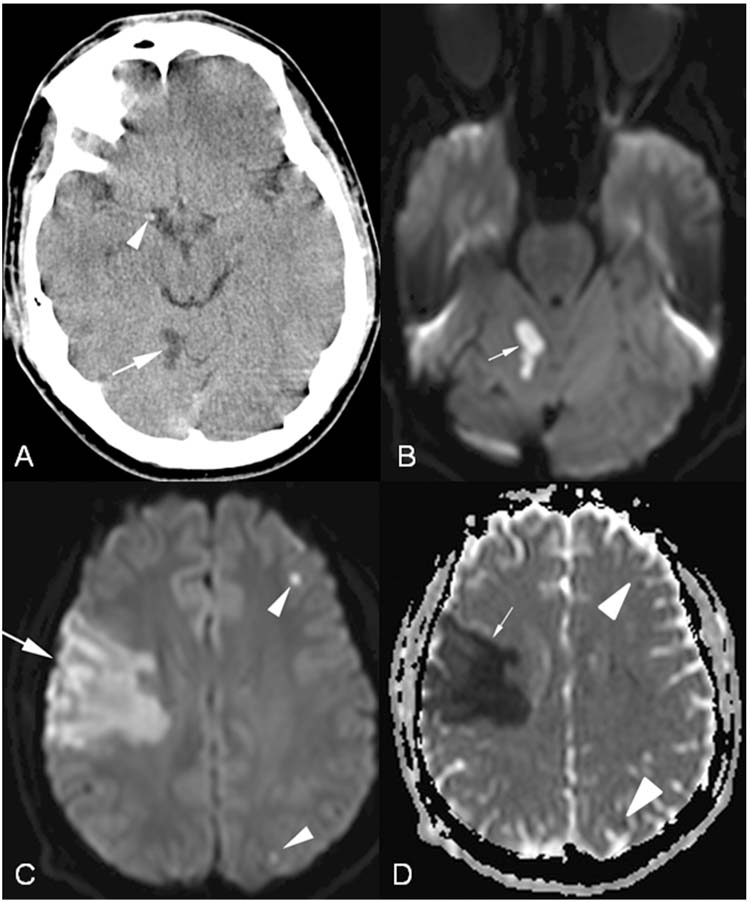
FIGURE 10.2 Cardioembolic stroke and hyperdense artery sign. A 15-year-old boy presented with severe headache, left hemiparesis, hemisensory loss, dysarthria, and neglect 2 days after an episode of dizziness and vomiting. (A), Axial head computed tomography demonstrating subacute cerebellar infarction (arrow) and hyperdense middle cerebral artery (arrowhead) representing acute clot. (B), Axial diffusion weighted imaging (DWI) with restricted diffusion of water (arrow) representing acute infarction corresponding to the area of hypodensity seen in (A). (C), Axial DWI demonstrating acute right middle cerebral artery infarction (arrow) as well as two other punctate areas of infarction on the left (arrowheads). The patient was found to have an intracardiac clot and was treated with heparin. (D), Axial apparent diffusion coefficient (ADC) map corresponding to the areas of restricted diffusion in (C) (arrow and arrowheads).
Even if subtle findings of AIS are present on HCT, brain MRI with diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) is more sensitive for the diagnosis of AIS. In acute AIS hyperintensity is seen on DWI which typically correlates in shape and size with the hypointensity seen on ADC map. These abnormalities on DWI and ADC appear within minutes of AIS onset and may last 7 to 10 days. Figure 10.2 demonstrates a HCT performed 2 days after acute stroke symptoms as well as DWI and ADC sequences on MRI. Other sequences on MRI that should be performed are contrast enhanced images and fluid attenuated inversion recovery sequences which can help to exclude demyelinating lesions as well as infectious processes. AIS may begin to enhance 5 days after the acute event.
In addition to the brain MRI, arterial imaging of the intracranial and cervical vessels should be obtained to evaluate for arteriopathy. Most often, arterial imaging is performed with magnetic resonance angiography (MRA), but CT angiography (CTA) can be performed if a child has a contraindication to an MRI/MRA. If MRA is suspicious for a cervical dissection or if one is suspected but is not seen, a contrast enhanced neck MRA may be helpful, or additional vascular imaging with CTA or even with conventional angiography may be required for a definitive diagnosis. Even in children with a known AIS risk factor such as congenital heart disease, arterial imaging should be obtained since many children with AIS have multiple risk factors.
■ INITIAL SUPPORTIVE CARE AND MANAGEMENT
The ischemic penumbra may become infarcted if perfusion is not restored quickly. Supportive care centers on minimizing metabolic demands of the tissue and avoiding additional insults like seizures, global hypoxia, hypercarbia, hyperthermia, hyperglycemia, or electrolyte disturbances. Thus, initial management principles in pediatric AIS focus on these items, although most data regarding supportive care is from adult AIS (12,13). In the United States, the American Heart Association as well as the American College of Chest Physicians have published guidelines for the management of pediatric stroke, as has the Royal College of Physicians (6,7,14). While there have been no clinical trials in childhood AIS except for stroke prevention in patients with SCD, the consensus guidelines are informed by prospective and retrospective cohort studies. Perhaps one of the most important early interventions is early initiation of speech, physical, and occupational therapy as well as other rehabilitation services in order to generate a recovery plan that fits the child’s needs.
Glycemia
Many experts agree that nondiabetic children with AIS should be kept euglycemic with dextrose-free intravenous (IV) fluids while hospitalized (4,7). According to one study, there is a greater mortality rate and a longer length of stay in hyperglycemic children when compared to nonhyperglycemic children for any illness (15). This may be even more pronounced in diabetic children with AIS. Glycemic control has been shown to improve outcomes of critically ill patients in the intensive care unit (ICU), and most children with AIS are admitted to the pediatric ICU, although this is center-specific. Nevertheless, despite strong consensus opinion recommending minimization of hyperglycemia, the degree of morbidity caused by hyperglycemia is unclear in childhood AIS (4).
Blood Pressure and Cerebral Perfusion Pressure
Acutely, when AIS is suspected, the child should be placed supine without pillows to promote blood flow to the injured brain tissue. Additionally, isotonic IV fluids like normal saline should be used at 1 to 1.5 times maintenance for age and weight as tolerated by the child’s cardiovascular system in order to augment cerebral perfusion. In rare cases, IV vasopressors may also be required to keep the mean arterial pressure at age-appropriate levels. It is postulated that hypertension is an adaptive response to cerebral ischemia. Unless the child with AIS presents with hypertensive crisis, urgency, or emergency, blood pressure should not routinely be lowered. However, if blood pressure is dangerously elevated, IV labetalol or nicardipine may be considered, based on the child’s heart rate, allergies, and other circumstances. The antihypertensive nitroprusside is contraindicated due to its cerebral vasoconstrictive effects. Current guidelines do not delineate precise blood pressure goals for childhood AIS, although many practitioners suggest aiming for mild hypertension with a systolic blood pressure greater than 75% for the child’s age.
Fever and Seizure Monitoring
Elevated body temperature and both convulsive and nonconvulsive seizures place significant metabolic demands on any ill child and must be managed immediately. Hyperthermia leads to a hypermetabolic state and portends a poorer prognosis than euthermia, according to animal models of AIS as well as prospective cohort studies in adult AIS (16,17). In an ischemic milieu, this effect may be magnified, causing neuronal injury at a faster rate because fever increases the demand for oxygen and glucose as the body attempts to reduce its temperature. Adult AIS guidelines suggest aggressive reduction of hyperthermia with cooling agents or antipyretics like acetaminophen, but it is still unclear if euthermia improves outcome in adult or pediatric AIS (12). Nevertheless, hyperthermia is associated with longer hospital stay and lower Glasgow Coma Scale in children with traumatic brain injury (18). Parallel studies need to be conducted in childhood AIS. Induced hypothermia is an emerging therapy in adult AIS trials. In the future, induced hypothermia may be studied in children, but at this time there is no recommendation to actively cool children with AIS.
Children with AIS who present with seizure or who have seizures should be treated with an antiepileptic drug, but it is not recommended that children with AIS receive antiepileptic drug prophylaxis in most cases. In a recent study, 22% of children in a cohort presented with seizures (19). Four of these children were monitored with long-term EEG for clinical reasons, and three of these four had electrographic seizures. The findings of this study suggest that nonconvulsive seizures may be underrecognized after acute childhood AIS, but there are no evidence-based recommendations for routine EEG monitoring in children. For children with continued seizures or altered mental status, continuous EEG monitoring may be helpful.
Intracranial Pressure Monitoring and Treatment
Large infarcts, particularly large middle cerebral artery territory infarcts, are often accompanied by significant cytotoxic edema, midline shift, and mass effect on the ventricles and may require urgent treatment for increased intracranial pressure (ICP). Symptoms of increased ICP may include headache, confusion, depressed mentation, worsening of initial stroke deficits, or coma with herniation. Thus signs and symptoms of increased ICP should be closely observed in a dedicated ICU setting during the first 72 to 96 hours after a large infarct, the peak time for swelling after AIS. Children have less brain atrophy than do adults and therefore may have less of an “anatomic buffer” against brain edema than adults. According to a pilot study evaluating craniectomy and duraplasty for refractory intracranial hypertension in children with traumatic brain injury, timely surgical decompression appeared to be both lifesaving and function-sparing (20).
More thorough evidence and guidelines are needed in the treatment of increased ICP in childhood AIS since current surgical practice is center-dependent and is informed by adult literature. The Royal College of Physicians does recommend early neurosurgical consultation for children with AIS and deteriorating consciousness or other signs of increased ICP (14). A meta-analysis pooling data from 3 adult randomized controlled trials on the topic showed a 71% mortality for medically managed adult AIS patients with early cytotoxic edema compared to 22% for those undergoing decompressive surgical intervention (21). The major caveat from this study is the morbidity: 35% of surgically managed adult AIS survivors were unable either to walk or perform activities of daily living without assistance, compared with 7% of adult AIS patients managed medically (22). However, in a pediatric series of 10 children with malignant middle cerebral artery infarction and Glasgow Coma Scales ranging from 3 to 9, outcome was moderately good following surgical decompression. All 3 who did not receive decompressive craniectomy progressed to brain death. However, the 7 children who had decompressive craniectomy had improvement of their mental status and walked independently at follow-up despite residual hemiparesis (23).
■ ETIOLOGIES OF PEDIATRIC AIS
Cardiac Sources
Heart conditions that have been associated with childhood AIS include arrhythmias, cardiomyopathies, congenital heart disease, valvular heart disease, Kawasaki disease, extracorporeal membrane oxygenation, cardiac surgery, and cardiac catheterization (7). Evaluation of the child’s heart for cardiac sources of emboli including valve abnormalities, vegetations, structural heart disease, or thrombus in the ventricles, atria, or even left atrial appendage should be performed in all children with AIS, particularly if there are strokes in multiple arterial distributions suggesting an embolic source (Figure 10.2). In most pediatric stroke cohorts cardiac disease is identified as a risk factor in a sizeable minority (24), but this estimate was as high as 69% in one series (8). Regardless, most children with AIS due to cardiac emboli already carry a diagnosis of congenital heart disease (25).
For those in whom the mechanism of AIS is unknown, a transthoracic echocardiogram (TTE) with a “bubble study” with agitated saline to look for right-to-left shunting, is usually the initial cardiac imaging modality. If the TTE fails to show any evidence of thrombus, valve abnormalities, vegetations, or other abnormalities, but a cardiac source is still suspected, a transesophageal echocardiogram (TEE) may be indicated. Theoretically, a patent foramen ovale (PFO) can lead to AIS via paradoxical emboli. If a clot were to form in a deep vein and move to the right atrium, it could cross into the left atrium if there is right-to-left shunting and thereby enter the systemic arterial circulation. However, results of a recent clinical trial did not demonstrate a decreased risk of recurrent stroke with PFO closure when compared to medical management (26). The management of AIS with a PFO found on TTE or TEE, especially without evidence of deep venous thrombosis on Doppler ultrasound of the extremities, is unclear at this point in time in both children and adults. Of course, if vegetations are found on the valves in a toxic-appearing child with AIS, infective endocarditis is the likely diagnosis and broad-spectrum antibiotics should be administered as quickly as possible. Blood cultures should be drawn if infective endocarditis is suspected.
Cerebral Arteriopathy
Cerebral arteriopathy is a common, major risk factor for childhood stroke, occurring in as many as 80% of children with AIS (4,27). Not surprisingly, with a recurrence rate of up to 66% at 5 years, it is also a predictor of poor short-term outcome (4,28).
Focal Cerebral Arteriopathy of Childhood and Postvaricella Arteriopathy
FCA, the most common type of arteriopathy seen in children with stroke, is characterized by a unilateral or bilateral focal or multifocal stenosis of the large and/or medium-sized intracranial arteries (Figure 10.3D) that is not attributed to specific diagnoses like moyamoya, arterial dissection, vasculitis, or postvaricella angiopathy (5). The most common location of stenosis is the distal ICA or proximal MCA or ACA, and infarction of the basal ganglia or internal capsule are common locations for the resulting stroke. The stenoses may improve with or without the artery fully returning to normal caliber and size. The stenosis and stroke can be monophasic; however, the stenoses may increase in number, tightness, or length over weeks to months leading to additional strokes and clinical deficits (29). The cause and pathogenesis are unknown, but FCA is likely a heterogeneous illness (5). Some experts believe that the arteriopathy may be due to invasion of the arterial wall by a pathogenic organism (5,29). A search for infectious agents such as varicella, HIV, syphilis, enteroviruses, and even Borrelia is often performed as soon as FCA is suspected. A similar radiologic and clinical picture has been associated with varicella infections, termed postvaricella arteriopathy. For that diagnosis, a varicella infection must be documented within 12 months of the acute AIS, and this may be less common since the advent of the varicella vaccine. A retrospective review of varicella in childhood AIS supports this parainfectious theory since 3-fold more children with AIS had a varicella infection within 1 year when compared to those without childhood AIS (30). Of 525 cases of childhood AIS in the International Pediatric Stroke Study, recent upper respiratory infection had a predictive odds ratio of 2.36 (95% confidence interval [CI], 1.05–5.27) for FCA (5). Therefore, future research for FCA therapy may focus on use of antiviral, antimicrobial, and antiinflammatory agents.
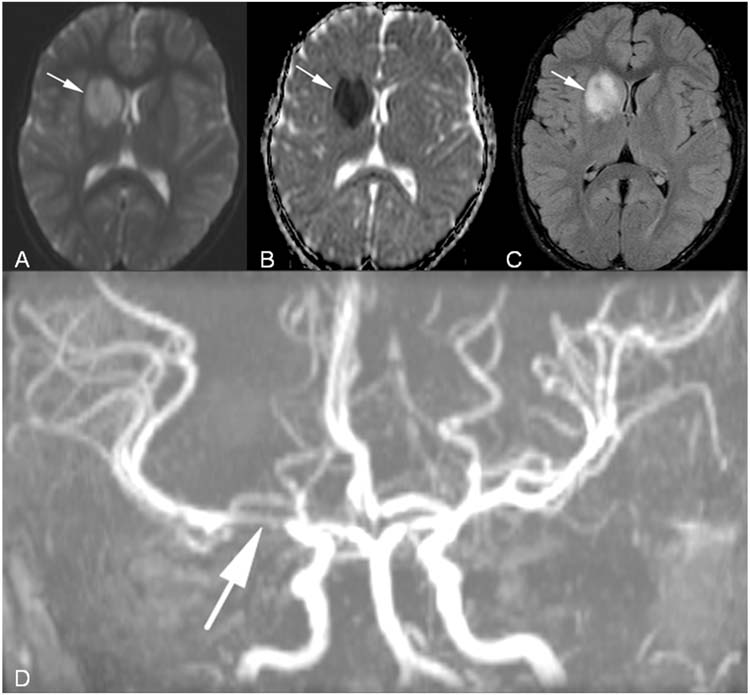
FIGURE 10.3 Focal cerebral arteriopathy of childhood. A 10-year-old girl presented with left facial weakness and left hemichorea. (A), Axial DWI with acute right basal ganglia infarction (arrow). (B), Axial ADC map corresponding to the area of infarction (arrow). (C), Axial fluid attenuated inversion recovery (FLAIR) image with hyperintensity in the right basal ganglia representing infarction (arrow). (D), MRA with focal stenosis of right middle cerebral artery (arrow).
Moyamoya Disease and Syndrome
Translated from Japanese literally as “smoky smoke,” moyamoya disease is a noninflammatory vasculopathy that is the second most common childhood arteriopathy (5,31). The name comes from its characteristic appearance during conventional cerebral angiography, like the puff of smoke from a cigarette, representing a nebulous pattern of contrast inside progressively smaller, fragile collateral arteries that form as a result of progressive occlusion of both ICAs (Figure 10.4) (31). More common in women than men and in children of Asian descent than in those from other races, idiopathic moyamoya disease classically presents with TIA, AIS, or less commonly with intracerebral or subarachnoid hemorrhage. There are two separate incidence peaks: one around age 5 years and another in the mid-40s. Other presentations include chorea or headache.
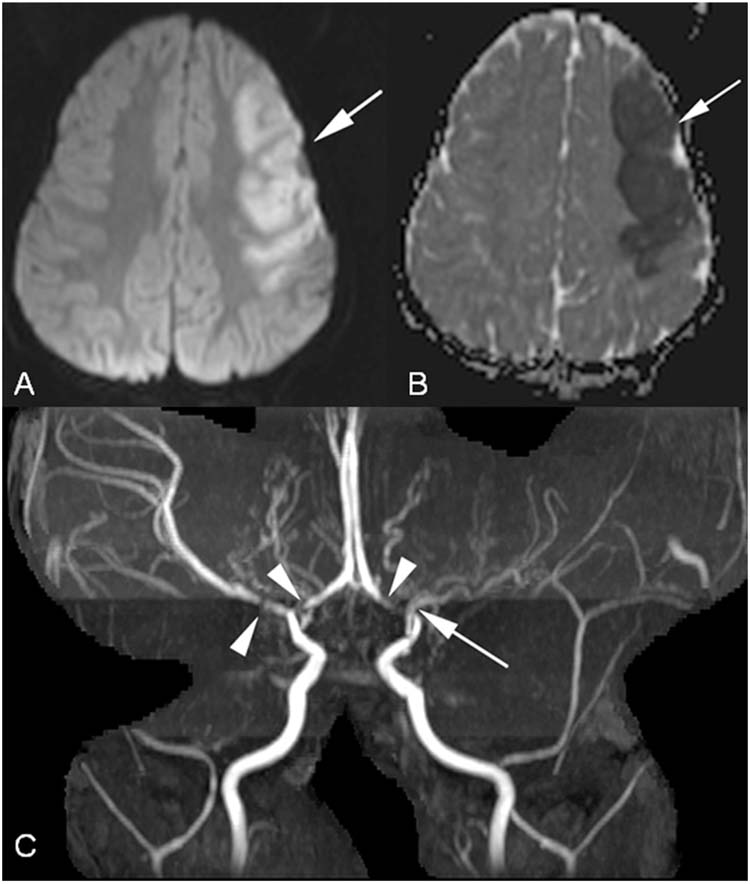
FIGURE 10.4 Moyamoya disease. This 2-year-old healthy male presented with a right sided complex partial seizure. (A), Axial DWI image with acute left middle cerebral artery stroke (arrow). (B), Axial ADC map corresponds to area of abnormality on DWI (arrow). (C), MRA demonstrates severe stenosis of the left middle cerebral artery (arrow) and stenoses of the bilateral supraclinoid internal carotid arteries involving both A1 segments of the anterior cerebral arteries and the right middle cerebral artery (arrowheads).
Moyamoya syndrome, unlike the idiopathic disease, is secondary to an associated condition such as SCD, trisomy 21, Williams syndrome (chromosome 7q deletion), neurofibromatosis type 1 (chromosome 17 mutation), and Alagille syndrome, or even cranial irradiation, regardless of dose or duration (31).
While in adults moyamoya is usually considered a bilateral disease, some children may have unilateral moyamoya and even involvement of the posterior circulation (Figure 10.5). To date, there is no known acute therapy to reverse the primary disease process, but experts recommend that children with AIS or TIA from moyamoya receive aggressive IV hydration and oxygenation as well as avoid hyperventilation and hypotension (31). Aspirin should be given to lessen the likelihood of microthrombi formation at sites of arterial stenosis. In most patients with AIS or TIA from moyamoya, a neurosurgical revascularization procedure should be considered because there is a high risk of recurrent AIS, and risk of intracerebral or subarachnoid hemorrhage increases over time. There are both direct and indirect bypass procedures available to help improve blood flow to the affected cerebral hemisphere(s). The indirect neurosurgical bypasses, such as encephaloduroarteriomyosynangiosis (EDMS) and encephaloduroarteriosynangiosis (EDAS), have demonstrated adequate collateralization with resulting resolution of symptoms in as many as 84% of patients (32,33). Direct bypass procedures, such as a superficial temporal artery-to-MCA anastomosis, have also shown effective collateralization and resolution of symptoms but may be more technically challenging than EDMS or EDAS, especially in children (34). There are no randomized prospective trials comparing surgical techniques, and hence the best strategy for revascularization remains uncertain and may depend on factors like surgeon experience with each procedure and the patient’s size and vasculature. Additionally, many patients with moyamoya develop intractable migraines; some experts believe that calcium-channel blockers may not only ameliorate these headaches but may also be effective in reducing both the frequency and the severity of refractory TIAs (31). In preparation for a revascularization surgery, a conventional angiogram of both the external carotid arteries and ICAs is usually required. When possible, revascularization is performed weeks after an acute stroke or a flurry of TIAs to minimize the perioperative stroke risk, but in patients with frequent symptoms, waiting may not be possible. Additionally, sedation or anesthesia procedures must be performed carefully to avoid hyperventilation and hypotension that can lead to additional infarction.
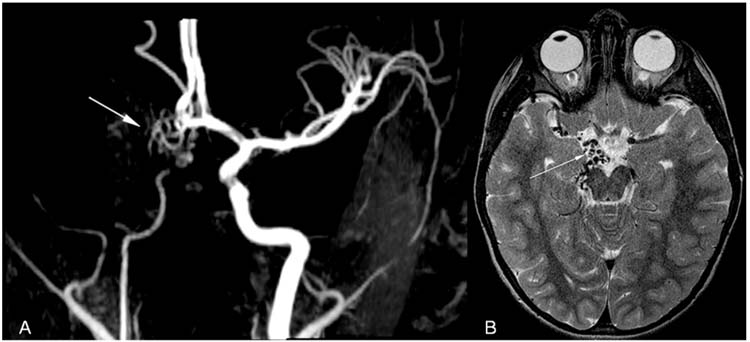
FIGURE 10.5 Unilateral moyamoya disease. This 3-year-old healthy female child presented with left hemichorea. (A), MRA demonstrates unilateral moyamoya disease on the right (arrow). (B), Axial T2-weighted MRI with multiple collaterals (arrow) typical of moyamoya disease.
Sickle Cell Disease
When compared to the general pediatric population, children with untreated SCD are at an alarming 100-fold increased risk of stroke because of sludging from sickled red blood cells and from arteriopathy (35). Red blood cells can sickle in patients with SCD in the setting of dehydration, infection, acidosis, or deoxygenation. Sickled cells can adhere tightly to endothelium and cause thrombus formation and AIS. About 12% of all SCD-related deaths are associated with an AIS event, according to one study. The cumulative risk of stroke for children with SCD increases with age, and by age 20 years, greater than 20% will have evidence of stroke-related damage on brain MRI (36). Besides AIS, neurologic sequelae of SCD in children may include primary intracerebral or subarachnoid hemorrhage, but AIS is more common. AIS often presents between age 2 and 5 years with hemiparesis, headache, or seizure. However, many children with SCD endure “clinically silent” infarctions, typically in the deep white matter, without overt neurologic deficits (37). Progressive occlusion of the small arteries supplying these territories can lead to a substantial accumulation of injury on MRI, which may result in cognitive deficits and behavioral dysfunction (37).
Children with symptomatic AIS from SCD often have a large vessel arteriopathy involving the supraclinoid ICA and its intracranial branches. This arteriopathy often progresses to ICA occlusions and formation of collateral vessels similar to the classic moyamoya appearance (31,37). Additionally, this large artery arteriopathy in children with SCD can lead to AIS in an anterior watershed pattern or lead to the formation of aneurysms, principally in the vertebrobasilar system (38). If symptomatic, extraordinarily large, or with precarious features, these aneurysms may need to be addressed either surgically or endovascularly.
Stroke risk for children with SCD can be forecasted by detection of increased blood flow velocity on transcranial Doppler ultrasonography (TCD), with high-risk children having an annual stroke risk of up to 10% per year. All 3 sets of pediatric stroke guidelines recommend that all children over 2 years of age with SCD obtain an annual TCD; those at highest risk of AIS by TCD (having a markedly increased velocity of intracranial arterial blood flow) should get chronic transfusion therapy (6,7,14). According to a landmark multicenter, randomized controlled study, 130 children meeting TCD criteria for increased intracranial arterial velocities with no history of prior stroke were randomized to transfusions (simple or exchange) that reduced hemoglobin S to less than 30% of total hemoglobin (experimental group) versus no intervention (standard care group) (39,40). Chronic transfusion reduced the risk of a first stroke by over 90% compared to a risk of 10% per year in the standard care group (40). Additionally, those with abnormal TCDs should have routine MRI/MRA to evaluate for vasculopathy and silent infarcts.
In the acute stroke setting, urgent erythrocyte exchange transfusion or simple transfusion that reduces hemoglobin S percent to less than 30% of total hemoglobin is also recommended, together with administration of IV hydration, supplementary oxygen, and packed red blood cell transfusion if the total hemoglobin concentration is less than 10g/dL (7,14,41). Notably, this practice has not been studied in a randomized trial. Revascularization procedures are normally reserved for children with large vessel arteriopathy associated with SCD who have continued strokes despite maximal medical therapy, including long-term transfusion therapy and hydroxyurea (7). In children who cannot undergo chronic transfusion therapy or who are refractory to transfusions, hydroxyurea is sometimes used (7).
Arterial Dissection
Stroke from arterial dissection, a common cause of arteriopathy in children, is caused by a tearing of the inner wall of an artery in the head (affecting the intracranial vessels) or neck (involving the carotid or vertebral arteries). A “false lumen” pocket may form in the torn vessel and pool blood that can coagulate and then embolize distally, causing an AIS. A pseudoaneurysm may also develop as a result of the tear and also can lead to an AIS by reducing cerebral blood flow or by rupturing. Either minor or major trauma may be the initial insult to provoke an arterial dissection, but patients with connective tissue disorders such as Marfan syndrome or Ehlers-Danlos syndrome may be prone to developing spontaneous arterial dissections without trauma. Often a prior trauma is never identified, even in previously healthy children. Regardless, dedicated arterial imaging of the head and neck is essential if dissection is the suspected diagnosis. Besides focal neurologic deficits, headache is the dominant symptom in about half of all children with dissection (42).
In children the anterior circulation is more likely to be involved than the posterior circulation (42,43). A systematic, retrospective review of 79 studies and case reports published between 1964 and 2000 identified 118 children with stroke from dissection (42). Many of the reports found a significant male predominance for both anterior and posterior circulation dissection and attributed this to a higher incidence of trauma among boys (42). However, in the systematic review, 74% of children with anterior circulation dissections and 87% of posterior circulation dissections were male, and the male predominance remained even after excluding children with preceding trauma. Of children with anterior circulation dissections, 60% were intracranial, unlike adult data in which intracranial dissections are rare (42). Children with intracranial dissections were more likely to be idiopathic or spontaneous and less likely to have had preceding trauma than those with extracranial dissections (42). The most common location for posterior circulation dissection is the vertebral artery at the C1-C2 vertebral body level (Figure 10.6), the segment most susceptible to dissection in adults’ posterior circulation as well. Recurrent AIS and TIA were reported more frequently after posterior circulation dissections than after anterior dissection, yet of the 10% of children with one dissection who had a dissection in a second vessel, all were in the anterior circulation (42).
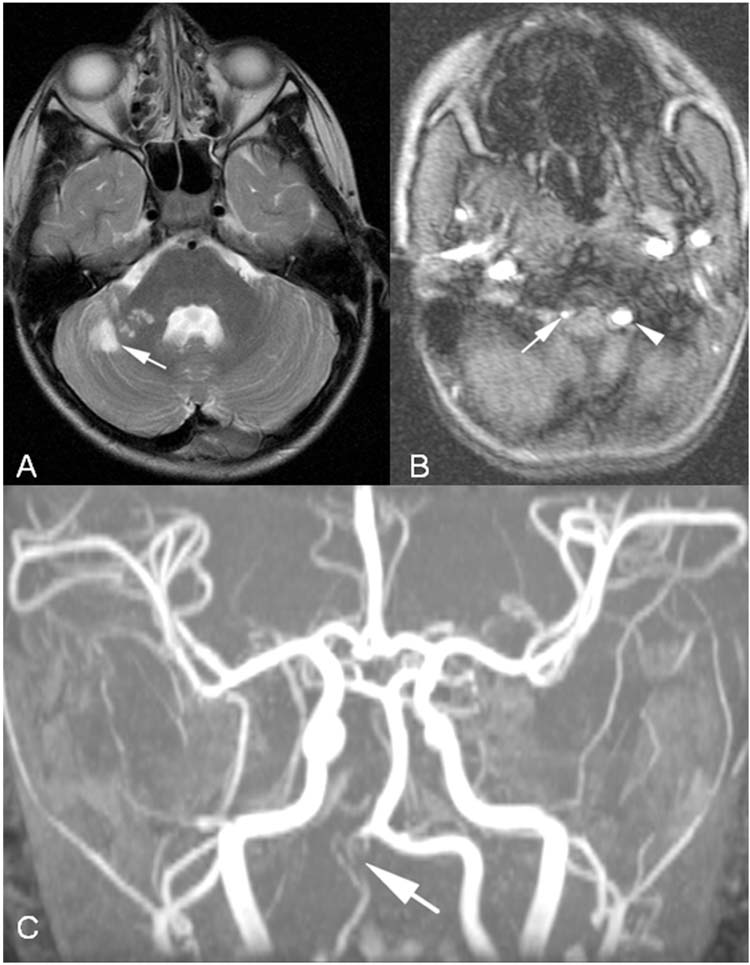
FIGURE 10.6 Verterbral artery dissection. This 8-year-old boy had acute onset of ataxia. Head computed tomography (HCT) was normal, and he was sent home without further workup. He returned to the emergency department 1 week later with new symptoms and was found to have multiple posterior circulation strokes. (A), Axial T2-weighted MRI with subacute right cerebellar strokes in the territory of the right superior cerebellar artery. (B), Axial MRA with narrow right verterbral artery (arrow) compared to normal caliber left vertebral artery (arrowhead). (C), MRA with diffuse narrowing of right vertebral artery (arrow).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


