
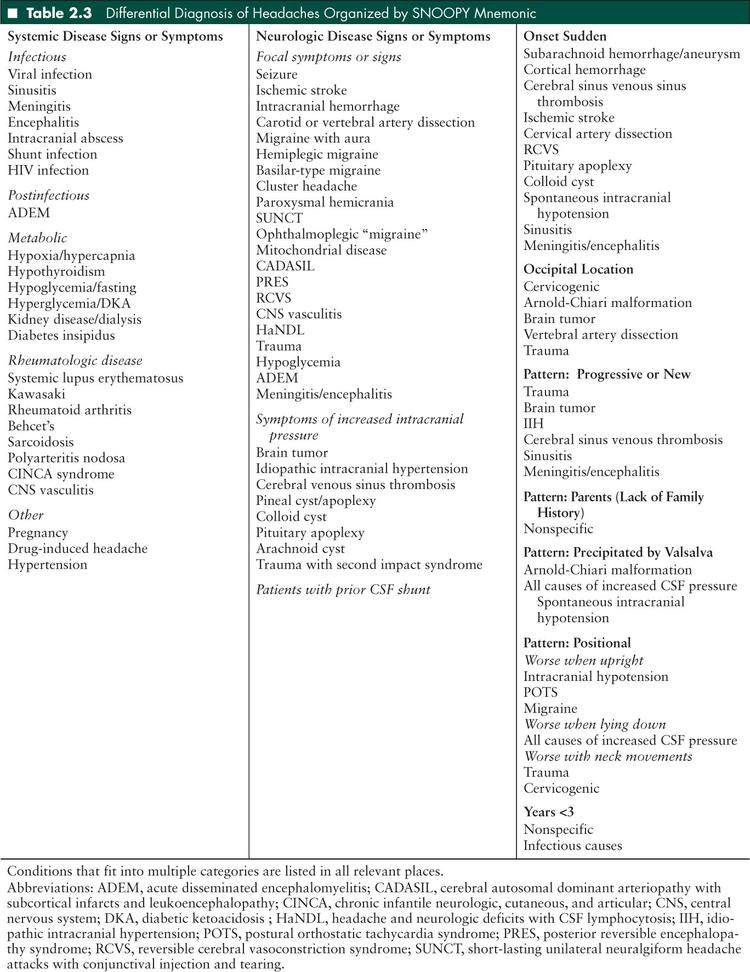
■ DIFFERENTIAL DIAGNOSIS OF HEADACHES BY PROMINENT HISTORICAL FEATURE
The red flags for serious headache conditions in children can be summarized by the mnemonic SNOOPY snoop4 (to remember the 4 Ps) secondary causes (1). The remainder of the chapter will be organized by this mnemonic. Some conditions fit into more than one category and are only listed once in the text. Table 2.3 provides a complete differential diagnosis.
Systemic disease signs or symptoms
Neurologic disease signs or symptoms
Onset sudden
Occipital location
Pattern: Progressive or new
Parents—lack of family history
Precipitated by Valsalva
Positional
Years <3
Systemic Disease Signs and Symptoms
Infectious
Infectious causes of headache are very common in children; studies have shown an infectious etiology in 30% to 60% of children presenting to the emergency department with a chief complaint of headache (2,3).
Viral Infection
Viral infections such as influenza, adenovirus, Epstein-Barr virus (EBV) and cytomegalovirus, and bacterial pharyngitis and otitis media are some of the most common causes of acute headache in children. Fever and tachycardia frequently produce a pounding headache that abates when the child’s temperature is lowered. Less common infections such as West Nile virus, Lyme disease, brucellosis, malaria, legionella, mumps, mycoplasma pneumonia, and tuberculosis of the central nervous system (CNS) may also cause headache (4–6). In these circumstances symptomatic treatment and antibiotics when indicated are usually all that are necessary. A type of chronic headache called New Daily Persistent Headache can start at the time of infections like EBV and the headache will persist for months to years, but this is rare (7). The presence of headache and seizure with fever is concerning, but does not always herald a severe condition, especially when the child has a history of febrile seizures and the seizure was generalized at onset.
Sinusitis
Sinusitis is another common cause of headache. It usually causes dull, periorbital pain and nasal congestion. However, when the symptoms are chronic or frequently recur, especially when the nasal congestion is mild, one should consider the diagnosis of migraine. Migraine frequently causes pain in the frontal and periorbital areas and involvement of the autonomic nervous system leads to nasal congestion (8). This overlap is further complicated because imaging studies done to evaluated headache often show incidental sinus disease, but treatment of the sinus disease does not affect the headache (9).
Acute isolated sphenoid sinusitis is seen in fewer than 3% of all cases of sinusitis. Although relatively rare, it is important to keep in mind as sphenoid sinusitis can present with subtle symptoms but can lead to multiple complications including intracranial spread (10). Headache is typically the most common presenting sign associated with sphenoid sinusitis and can present anywhere in the craniofacial region. The pain usually will increase over time and may interfere with sleep (11).
Meningitis, Encephalitis, Intracranial Abscess
Less common are intracranial infections like meningitis and encephalitis. These should be suspected when the child has a new severe headache with fever, nuchal rigidity, change in mental status, photophobia, phonophobia, nausea, pain with eye movements, rash, or abnormalities on neurologic examination. The headache may be anywhere on the head, but is usually holocranial, frontal, or occipital. The onset may be gradual or abrupt. Bacterial causes are the most dangerous, but viral and chemical causes are more common. Even rarer are intracranial abscesses which can arise in children with meningitis, otitis, mastoiditis or sinusitis, congenital heart disease, neurosurgical procedures, open head trauma, or immunocompromise. These may also cause focal neurologic signs or seizures. If intracranial infection is suspected, especially if there are any focal neurologic signs or symptoms, initial evaluation should include brain imaging prior to lumbar puncture (LP). Empiric antibiotic therapy should be started immediately (4,12,13).
HIV Infection
Headache can occur with HIV infection at almost any stage of the disease, from acute seroconversion to AIDS. Headache may be related to viral load, and especially in later stages of the disease, can be a sign of opportunistic infection (4).
Postinfectious
Acute Disseminated Encephalomyelitis
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating disease generally thought to be provoked by infection or vaccination. ADEM is most commonly a monophasic illness and can present with a wide range of neurologic signs and includes some degree of encephalopathy. The diagnosis is confirmed with magnetic resonance imaging (MRI), which typically shows T2 hyperintense white matter lesions. While the presenting signs of ADEM may be varied and nonspecific, one case series reported headache to be one of the most common presenting signs. In this series, headaches were reported as part of the prodromal phase as well as part of the encephalopathy associated with an episode of ADEM (14).
Metabolic Derangements
Many metabolic derangements can cause headaches in children. If any of these disturbances are suspected based on a patient’s history and exam, laboratory tests should be done to confirm the abnormality. Headaches should be temporally related to the metabolic disturbance in question and should improve as the abnormality is corrected.
Hypoxia/Hypercapnia
Headaches occur in conditions such as high altitude, diving, or sleep apnea. With high altitude, the headache is generally bilateral, frontal, and aggravated by exertion. It occurs above 2,500 meters within 24 hours of ascent and resolves within 8 hours of descent. Medical treatment of this disorder includes use of acetazolamide and possibly steroids (15). With sleep apnea, the typical headache is usually brief, bilateral, and occurs upon awakening. These children will often snore and have enlarged tonsils or signs of obesity on exam. If sleep apnea is suspected, a polysomnogram should be done (16).
Hypothyroidism
Hypothyroidism has been found to be a risk factor for daily headache, and about 30% of patients with hypothyroidism also have headaches. Headaches are typically described as bilateral and nonpulsatile, and occur within 2 months after the onset of hypothyroidism. The headaches should resolve within 3 months of adequate thyroid replacement therapy (15).
Hypoglycemia/Fasting
Hypoglycemia and fasting are 2 other common causes for headache in children. It is important to note that headaches that occur while fasting may be multifactorial as dehydration and possibly caffeine withdrawal may contribute to the pain (13).
Hyperglycemia/Diabetic Ketoacidosis
Cerebral edema occurs in 0.3% to 1% of children with diabetic ketoacidosis and can occur before or during treatment of diabetic ketoacidosis. Headache can be an early sign of cerebral edema and should be monitored with along with mentation and vital signs (17).
Kidney Dysfunction, Including Hemodialysis
Patients with severe underlying renal disease may complain of headaches, but it may be related to treatment with hemodialysis. In fact, 70% of patients getting hemodialysis complain of headache in more than half of their treatments (15). It has been reported that headaches resolve completely in these patients after successful transplantation and completion of hemodialysis treatments.
Diabetes Insipidus
Along with the characteristic symptoms of polyuria and polydipsia, headache can be a common complaint in children with diabetes insipidus. As sodium levels fluctuate in the setting of diabetes insipidus and its subsequent treatment, headaches can occur in the setting of initial hypernatremia or with aggressive treatment causing hyponatremia and cerebral edema.
Rheumatologic Disease
Headache is a frequent complaint in patients with rheumatologic disease. In fact, one study found that more than half of all systematic lupus erythematosus (SLE) patients reported either migraine or tension-type headache. There was no association found between type or severity of headache and SLE disease status, including whether the CNS was involved (18). Kawasaki disease is a disease that is characterized by persistent fever, conjunctivitis, rash, lymphadenopathy, and abnormalities in the lips and oral mucosa. The disease is characterized by vasculitis and headaches may occur. Patients with rheumatoid arthritis can have arthritic changes in their cervical vertebrae or atlantoaxial instability with associated headache. Headaches have also been reported in Behcet’s disease with the etiology being aseptic meningitis or benign intracranial hypertension. Other rarer rheumatic disorders in children that have associated headaches are sarcoidosis, polyarteritis nodosa, and CINCA syndrome (chronic infantile neurologic, cutaneous, and articular syndrome). Secondary CNS vasculitis may cause headache in patients with rheumatologic disease (18).
Other Systemic Causes of Headache
Pregnancy
Headaches, both immediately before and after delivery, may be one of the most sensitive markers for severe complications such as eclampsia, sinus venous thrombosis (SVT), and posterior reversible encephalopathy syndrome (PRES). In order to determine the etiology of a headache, a careful history must be obtained.
Eclampsia occurs when a patient with preeclampsia (hypertension, proteinuria, and edema) develops generalized seizures. Though eclampsia typically occurs prior to delivery, it has also been reported in the postpartum period and can even occur more than 48 hours after delivery. One of the most sensitive presenting signs of eclampsia is headache, which has been reported in up to 87% of cases (19). Other associated symptoms may be nonspecific and include visual changes, nausea and vomiting, and abdominal pain.
Other serious causes of postpartum headaches include headaches secondary to PRES and cerebral sinus venous thrombosis. PRES is typically characterized by visual disturbance, seizure, encephalopathy, and hypertension in addition to headache. Patients with cerebral sinus venous thrombosis report that headache is the most common and earliest symptom (20). These headaches are typically gradual in onset. If associated with increased ICP, they are typically characterized as dull, severe, generalized pain that can worsen with Valsalva and lying down. To differentiate the above etiologies, brain MRI should be done. Vascular imaging may also be needed if a venous thrombosis is suspected.
Another cause of a postpartum headache is a low-pressure headache secondary to an inadvertent dural leak from epidural anesthesia during delivery. This type of headache should be differentiated based on history as these headaches are positional and worse when in an upright position (19).
Drug Induced Headache
Headaches can be associated with a variety of medications, with one of the most common being hormonal contraceptives. Other medications may cause headache due to aseptic meningitis which may be associated with meningismus. Examples of these medications include ibuprofen, intravenous immune globulin, penicillin, trimethoprim, and intrathecal agents. Evaluation of this type of headache may include removal of offending drug and subsequent observation. If meningismus is present, LP may be considered for further workup (16).
Alternately, medication overuse headache is a daily headache that occurs in the context of chronic analgesic use (greater than 3 times per week for at least 3 months), usually initiated for treatment of frequent migraine or tension-type headaches. Typically there are no other associated neurologic signs and the exam is normal. Evaluation includes withdrawal of offending agent with subsequent reevaluation (16).
Hypertension
Hypertensive crisis or encephalopathy may present with acute, severe headaches. While significant elevation of blood pressure may result in headache, typically mild to moderate hypertension does not cause severe headaches (21). See discussion of PRES below regarding hypertensive encephalopathy.
Neurologic Disease Signs and Symptoms
Headache With Focal Neurologic Symptoms or Signs
Seizure
The relationship between headache and epilepsy is complex, and they are comorbid more often that would be expected based on the prevalence of each condition. The pathophysiology of both conditions is related to brain hyperexcitability. There are many ways in which seizure and headache episodes can overlap, including postictal headache, preictal headache, headache that leads to seizure, and seizures with headache as a primary manifestation. Focal seizures can cause focal symptoms or abnormalities on exam, while primarily or secondarily generalized seizures cause loss of consciousness.
Surveys of patients with epilepsy have found that 12% to 52% experience postictal headache, which is defined as headache that starts within 3 hours of a seizure and resolves within 72 hours. It is usually moderate to severe in intensity, and can have features of migraine or tension-type headache. Patients with epilepsy who have migraine headache interictally and those with more severe and long-standing epilepsy are more likely to have postictal headache. Longer seizures and generalized tonic-clonic seizures are more likely to provoke postictal headache. Both analgesics and triptans have been used effectively to treat these headaches. It is not known if antiepileptic medications used for headache prevention (topiramate, valproic acid, etc) have any effect on postictal headache (22). Preictal headaches are less commonly reported (23).
Migralepsy, defined as an episode of migraine with aura progressing to an epileptic seizure, has been reported in about 50 cases. Other authors have criticized that those reports actually described occipital lobe seizures. There are some historical features that can distinguish migraine aura from ictal visual changes. Migraine visual aura usually starts as small flashes of lights or zigzag line which progress over 5 to 30 minutes, often followed by a scotoma. It is usually white or uncolored but can be colorful (24). The hallucinations of occipital lobe epilepsy are usually colorful and circular, develop within a few seconds, widen and multiply, and last a total of a few minutes (25).
There are two idiopathic focal epilepsy syndromes of childhood where headache is a major manifestation of the seizure itself. Idiopathic Childhood occipital epilepsy of Gastaut, also called late onset childhood occipital epilepsy, has been described in children aged 3 to 16 years who have brief visual hallucinations that may progress to other visual or ocular symptoms. Ictal or postictal headache may be orbital or migrainous. The electroencephalogram (EEG) shows paroxysms of spike-wave or sharp discharges in the occipital region with eye closure (26). Early onset benign childhood seizures with occipital spikes, also call Panayiotopoulos syndrome, is a syndrome of infrequent, usually lengthy, nocturnal seizures in children with nausea, retching, and vomiting. Other autonomic manifestations include pallor, pupillary changes, cardiorespiratory and thermoregulatory alterations, incontinence of urine or feces, and hypersalivation. Headache may be present, leading to the misdiagnosis as “atypical migraine.” The seizure may secondarily generalize or progress to syncope. The EEG typically shows multifocal, high amplitude sharp and slow wave complexes. Children tend to have a small number of seizures over their lifetime and therefore may not need antiepileptic therapy (27,28).
Ischemic Stroke and Intracranial Hemorrhage
Headache, focal neurologic deficits, and altered mental status can be presenting signs of ischemic stroke or intracranial hemorrhage.
Cervical Artery Dissection
Dissection of the carotid or vertebral artery usually causes head and neck pain followed days to weeks later by symptoms of ischemia; ipsilateral Horner syndrome may accompany carotid dissection.
Primary Headache Disorders
These conditions will be discussed below in more detail.
Briefly, patients with migraine with aura can have focal abnormalities including visual, speech, and sensory disturbances. Patients with hemiplegic migraine have focal weakness associated with headache. Basilar-type migraines cause brainstem dysfunction including vertigo, visual disturbances, bilateral sensory symptoms, and ataxia. Rarely migraines are the presenting sign of progressive genetic or metabolic conditions.
Trigeminal autonomic cephalgias such as cluster headache, paroxysmal hemicrania, and SUNCT (short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing) have prominent autonomic features including unilateral ptosis, conjunctival injection, lacrimation, and rhinorrhea. These conditions can be secondary to pituitary or vascular abnormalities, so children with these conditions require MRI to evaluate for a secondary cause.
Ophthalmoplegic “Migraine”
This migraine variant is defined as at least two episodes of headache accompanied or followed within 4 days by paresis of one or more of the third, fourth, and sixth cranial nerves. The most recent International Classification of Headache Disorders does not include this as a type of migraine, but recommends imaging to rule out parasellar, orbital fissure, and posterior fossa masses and to look for gadolinium-enhancement of the cranial nerves (21).
Mitochondrial Disease
Headaches and migraines are frequently seen in patients with known mitochondrial disease, but the pathophysiology is still unknown (29,30). As most patients with significant mitochondrial disease have notable neurologic dysfunction and effects on multiple organ systems, screening for mitochondrial disease in patients with isolated headache is not indicated (13). Headache is one of the most common complaints, and may be the presenting symptom, with mitochondrial encephalopathy lactic acidosis and strokelike episodes (MELAS) (31,32). MELAS is a multisystem disorder characterized by strokelike episodes, encephalopathy with seizures or dementia, and lactic acidosis. Other common features of MELAS include vomiting, short stature, hearing loss, and muscle weakness. MELAS is associated with mutations in mitochondrial DNA and is transmitted by maternal inheritance. It typically presents in childhood after a normal early development (33,34). Mutations in the POLG gene have also been associated with headache (35).
Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an underrecognized condition caused by mutations in the Notch3 gene. While this entity is typically seen in adults, there have been case reports in children. Clinically, patients typically present with migraines first and then recurrent episodes of stroke or transient ischemic attacks (36). Other clinical features include the development of cognitive deficits and dementia in more than half of patients and neuropsychiatric symptoms such as mood disorders in 25% to 30% of patients (37). Apathy has been reported as one the most common neuropsychiatric problems associated with CADASIL and can occur independent of depression (38).
Since CADASIL is inherited in an autosomal dominant fashion, it should be suspected in patients with a strong family history of migraines and stroke at an early age in the absence of vascular risk factors. Although migraine with aura is most common, atypical patterns such as aura without headache, prolonged aura, and hemiplegic migraine may be seen (39). Brain MRI is helpful in diagnosis as T2 hyperintense lesions in the deep and periventricular white matter and anterior temporal poles are commonly seen. Definitive diagnosis can be made with genetic testing (screening of the Notch3 gene) or by skin biopsy as small vessel arteriopathy with granular osmiophilic material is diagnostic.
Posterior Reversible Encephalopathy Syndrome
PRES is also called reversible posterior leukoencephalopathy syndrome, and encompasses what was previously called hypertensive encephalopathy. Patients typically present with seizure, visual disturbances, headache, and altered mental status; a review of pediatric cases showed that 76% had 3 of those 4 symptoms (40). Seizures may be focal or generalized, and may lead to status epilepticus. Visual disturbances range from blurred vision to cortical blindness. Headaches are often severe. Mental status may wax and wane, and impairment may be so severe as to necessitate intubation. Nausea, vomiting, papilledema, and focal neurologic findings such as cranial nerve palsies may also be present (41). Stroke and hemorrhage are infrequent complications. There is substantial overlap with reversible cerebral vasoconstriction syndrome (RCVS) in that the same agents may provoke either, and many patients have evidence of both conditions (42).
PRES is associated with acute hypertension, preeclampsia and eclampsia, immunosuppressants (including tacrolimus, cyclosporine, and steroids), bone marrow or solid organ transplantation, chemotherapy, and autoimmune disease (41). Case series of children have also reported an association with transfusion, HIV infection (40), poststreptococcal glomerulonephritis (43), hemolytic uremic syndrome, and intraabdominal neurogenic tumors (44).
The name of the syndrome refers to the typical pattern seen on imaging of edema in the parietal and occipital white matter. However, gray matter is often involved, and frontal cortex, basal ganglia, corpus callosum, brainstem, and cerebellum can be involved (44,45). While severe PRES can be detected on computed tomography (CT), MRI is more sensitive. Lesions are hypointense on T1-weighted images, hyperintense on T2-weighted and fluid-attenuated inversion recover (FLAIR) recovery images, isointense or bright on diffusion-weighted imaging (DWI), and bright on apparent diffusion coefficient (ADC) map images, consistent with vasogenic rather than cytotoxic edema (43,45). Sometimes the lesions enhance with gadolinium, but this is usually not a striking feature. A few cases reported in the literature have had repeated follow-up MRIs showing incomplete resolution less than 1 week after onset with normalization later (45). If PRES is diagnosed, magnetic resonance angiography (MRA) should also be performed to look for reversible cerebral vasoconstriction given the frequent clinical overlap, and the use of calcium channel blockers to treat the latter. LP is not needed for the diagnosis, but may show elevations in opening pressure up to 50 cm of water (40). Cerebrospinal fluid studies are usually normal, but a mild elevation in protein may be seen (41). EEG can be normal or show focal or diffuse slowing, focal epileptiform discharges, or seizures (43).
Treatment should be aimed at withdrawal of the causative agent when possible, aggressive management of hypertension, and symptomatic treatment of seizures. There has not been any report of how to treat the associated headache, but medications that could exacerbate hypertension should be avoided. While the name implies that the condition will resolve, permanent changes have been reported in up to one quarter of adult cases, and in a few pediatric cases. Recurrent PRES has also been described (45).
CNS Vasculitis
While secondary CNS vasculitis may be associated with systemic rheumatologic disease, primary CNS vasculitis is rare in the pediatric population and is generally accompanied by other signs of CNS inflammation or ischemia (13). Acute severe headache, focal neurologic deficits, and cranial nerve abnormalities are all common presenting features of primary CNS vasculitis in children. If CNS vasculitis is suspected, further evaluation with an LP may be pursued as studies in adults and children have shown the presence of either elevated protein or a pleocytosis in the cerebrospinal fluid of patients with vasculitis (18). Although MRA may be an additional diagnostic tool for detecting CNS vasculitis, brain biopsy is the gold standard for diagnosis. Serologic studies for evaluation of a possible systemic rheumatologic disorder may also be done as part of the workup if vasculitis is suspected.
Syndrome of Transient Headache and Neurologic Deficits With (CSF) Lymphocytosis (HaNDL)
Syndrome of transient headache and neurologic deficits with Cerebrospinal Fluid (CSF) lymphocytosis is characterized by recurrent episodes of neurologic deficits accompanied or followed by moderate to severe headache. The condition was previously called pseudomigraine with lymphocytic pleocytosis. Sensory symptoms are more common than aphasia, motor deficits, or visual changes, and usually last several hours. Patients usually have no history of headaches before these episodes start, but one-third have a viral prodrome. When performed, LP shows CSF lymphocytic pleocytosis (> 15 cells/µL), elevated CSF protein, and sometimes elevated opening pressure; cultures and microbiologic studies are usually normal, though there was one reported case with elevated human herpesvirus 6 (HHV-6) immunoglobulin M when symptoms developed after a viral prodrome (46). Nausea, vomiting, fever, and papilledema may be present. Routine imaging including MRI DWI is usually normal, but perfusion studies may show hypoperfusion in the affected area (47). EEG may show focal abnormalities consistent with symptoms. One case report describes resolution of symptoms with intravenous dexketoprofen (47). Patients are asymptomatic between episodes (21), and the recurrences usually cease within a few months. Multiple case series have been reported, including children as young as 7 years old (48).
Headache With Signs/Symptoms of Increased ICP
Signs of increased ICP include altered consciousness, vomiting, visual problems including blurred vision and diplopia, worsening of headache when supine, bending forward, or coughing, and pulsatile tinnitus.
Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension (IIH), also called pseudotumor cerebri, is elevated ICP without evidence of structural abnormality in the brain. The headache is usually dull and daily, sometimes accompanied by migrainous features, but can present more acutely. Historical features that suggest IIH includes transient obscurations of vision, blurred vision or diplopia, pulsatile tinnitus, and retro-orbital pain (49). Young children may also show neck stiffness, irritability, somnolence, and ataxia (13). Physical examination should be normal other than papilledema, though the absence of papilledema does not rule out IIH.
The pathophysiology of IIH is not well understood, but is believed to be related to an imbalance between CSF production and resorption. Obese adolescent girls and young women are most commonly affected, but in young children boys and girls are equally affected and there is no association with obesity. Vitamin A, retinoic acid, nitrofurantoin, withdrawal of steroids, thyroid replacement in children with hypothyroidism, and hyperparathyroidism have been associated with IIH. Previously found links with tetracycline, minocycline, oral contraceptives, and pregnancy have been called into question (49).
Particularly in young children the diagnosis is one of exclusion, and a thorough evaluation should include MRI to look for structural abnormalities and signs of infection, and magnetic resonance venography (MRV) to look for thrombosis of the cerebral veins. Magnetic resonance (MR) findings consistent with IIH include an empty sella, flattening of the globes, and stenosis of the cerebral veins. Patients should also have a thorough ophthalmologic evaluation, as decreased visual acuity and visual field loss are often not appreciated or reported by the patient (50).
Diagnosis is made by LP with measurement of opening pressure in the lateral decubitus position. However, the upper limit of normal CSF pressure is not well established in children. One prospective study proposed a value of 28 cm water, but the subjects included in that study all had LP performed due to clinical symptoms, including many with the primary complaint of headache (51). Other studies have shown that adult patients with chronic daily headache, some of whom had stenosis of the venous sinuses, can have significant fluctuation in CSF pressure even when the opening pressure is within the normal range (52,53). Abnormalities in the CSF analysis should prompt testing for chronic infections like Lyme and inflammatory conditions, and preclude the diagnosis of IIH.
Treatment involves several steps. The LP itself can be therapeutic. When the CSF opening pressure is greater than 20 cm water and the history suggests this diagnosis, CSF should be drained to bring the closing pressure to 10 to 20 cm water. Development of low pressure headache after LP does not exclude the diagnosis of IIH. If the headache returns after LP, medical therapy with acetazolamide or topiramate should be initiated. Both of these decrease CSF production by inhibiting the action of carbonic anhydrase. Carefully evaluating for worsening of vision is important and for severe cases where vision is threatened, intravenous steroids can be a temporizing measure, but surgical management with optic nerve sheath fenestration may be necessary. Lumboperitoneal or ventriculoperitoneal shunts are done for extreme cases, but are generally avoided due to a high incidence of shunt failure (50). In obese patients, weight loss is the best long term strategy.
Pineal Cysts/Apoplexy
Pineal cysts are very common, seen on up to 4% of MRIs and 20% to 40% of autopsies. Usually cysts are found incidentally, and are asymptomatic and benign, but rarely they enlarge and become symptomatic. This enlargement can cause obstruction of the third ventricle and hydrocephalus which in turn causes paroxysmal or chronic headache, papilledema, and altered mental status. Pressure on the midbrain causes eye movement abnormalities. An extremely rare complication of pineal cysts and tumors, pineal apoplexy involves sudden hemorrhage into the pineal gland with resultant acute hydrocephalus and abrupt worsening of headache and mental status. Patients may have been symptomatic for months or even years before the acute worsening. A few children have been reported. The youngest was 9 months old. Management options including treatment of the hydrocephalus with ventriculoperitoneal shunt and aspiration or excision of the lesion (54,55).
Arachnoid Cysts
Arachnoid cysts are collections of CSF within the arachnoidal membrane and subarachnoid space. These can occur in many locations, including along the cerebral fissures, in the sella, and at the cerebellopontine angle. They are relatively common, with a prevalence of about 0.7%, and are often found incidentally when head imaging is done after trauma. Bilateral cysts are unusual, and can be associated with glutaric aciduria type I. Arachnoid cysts are usually asymptomatic but can produce symptoms including headaches, seizures, weakness, and hydrocephalus. Long-term most of them remain stable, but they can acutely enlarge and cause symptoms (56) or reduce spontaneously (57). Infrequently after head trauma the contents rupture, leading to subdural hygromas or intracranial hypertension (56). Most of the case series in the literature have been reported by neurosurgeons, and overall the majority of patients were reported to improve with surgery, especially when fenestration rather than shunting was performed (58). However, there is disagreement about whether all symptomatic patients should be referred for surgery (59).
Headache Associated With Intraventricular Shunts
Headache in a child with a CSF shunt is always worrisome because it can be a sign of shunt malfunction. Shunt malfunction occurs in as many as 50% within 2 years of placement. Patients usually present with nausea, vomiting, headache or irritability, and altered consciousness, but can also have seizures, weakness, cranial nerve abnormalities (especially VI), ataxic gait, and papilledema. Young children and infants can present with a bulging fontanel, splaying of the cranial sutures, or increasing head circumference. In most cases head imaging and a “shunt series,” x-rays to image the shunt tubing throughout its course, should be performed. Due to its speed and availability in emergency situations, head CT is usually the study of choice, but MRI is more sensitive for detection of transependymal flow. Ideally, the scan should be compared to a prior image from a time when the child was well.
Causes of shunt malfunction include:
Infection—Most common cause of shunt failure in the immediate postoperative period. Typically presents in the days to weeks after surgery. Persistent infection can lead to multiple shunt revisions within a short period of time. Fever is usually present.
Shunt misplacement—Rare. Presents with signs of increased ICP in the immediate postoperative period.
Obstruction—Relatively common. Can present days to years after surgery. Obstruction can occur at the ventricular catheter, valve, or distal catheter (60).
Overdrainage—Refers to situations where the shunt is removing more fluid than desired. Rarely overdrainage leads to extraaxial fluid collections in the immediate postoperative period, but more commonly the excessive drainage leads to “slit ventricle syndrome” years later. Patients with slit ventricles often present with recurrent attacks of vomiting and headache which improve with lying down, whereas patients with obstruction typically experience acute or progressive symptoms that are worse when the child is supine. Because patients with slit ventricle syndrome can have normal sized ventricles the diagnosis can be difficult; migraine medications or surgical revision of the shunt may be beneficial (61,62).
Loculation—Refers to noncommunicating fluid pockets within the ventricles. The shunt drains the fluid pocket to which it is connected, but the others can enlarge and create signs of hydrocephalus. Rarely patients can develop an enlarged, “trapped” fourth ventricle, and present with cranial nerve abnormalities from brainstem dysfunction (61).
Fracture, disconnection, or migration of the distal tubing—Typically presents with signs of local irritation around the tubing years after surgery. These patients may not have signs of increased ICP (60).
Abdominal complications—Ascites, pseudocyst, constipation, or perforation of an abdominal organ can all lead to hydrocephalus by preventing appropriate drainage from the shunt (61).
Patients with CSF shunts can also have primary headache disorders such as migraine headaches, but these must be diagnoses of exclusion. Given the high prevalence of primary headache disorders, these patients might have developed migraines even if they had never needed a shunt, or the hydrocephalus and shunting may have somehow lowered their threshold for migraine headaches (62). Once an ominous cause has been ruled out, the patient should be treated with migraine therapies (see below). Rarely, patients may complain of headache from allodynia at the shunt bubble or incision site on the scalp (S. Silberstein and W. Young, personal oral communication, 2010).
Onset Sudden
Headaches that are maximal intensity at onset, called thunderclap headache, raise concern for a serious underlying problem, especially a vascular cause like subarachnoid hemorrhage (42). Recurrent thunderclap headaches can be caused by repeated bleeds from aneurysm and by reversible cerebral vasoconstriction. When all other causes have been excluded the diagnosis Primary Thunderclap Headache has been described in adults, but there have been no reports of children with this condition.
Workup for Thunderclap Headache
The workup for Thunderclap Headache is much more aggressive than for other headaches, and should proceed stepwise until an etiology is found. The timing of these tests will depend on the condition of the patient and availability of testing at your institution, but should proceed as rapidly as possible as most causes of thunderclap headache can cause significant morbidity and mortality. This list is based on the author’s experience and review of the literature, but is not based on any formal recommendations.
Noncontrast head CT to evaluate for acute bleeding or early signs of ischemia.
LP to evaluate for bleeding or xanthochromia. Since it is difficult to differentiate a traumatic tap versus actual hemorrhage, make sure to check cell count in the first and last tubes collected. If the number of red blood cells is significantly elevated and relatively stable between the two tubes consider it positive for hemorrhage. Also measure the opening pressure. Opening pressure greater than 20 to 25 cm of water can be found with many conditions. Opening pressure less than 6 cm of water is diagnostic for intracranial hypotension (IH). If patient has focal neurologic deficits consider MRI before LP to evaluate for ischemia. If IH is suggested by history, the MRI should be done before LP, as LP may exacerbate the condition. If colloid cyst is suggested by history or found on imaging, LP should not be done.
MRI of the brain with diffusion-weighted sequences (looking for ischemia). If possible include both noncontrast and postcontrast sequences as IH is best detected with contrast. If the MRI shows ischemia, if reversible cerebral vasoconstriction is suspected, or if any of the above tests have shown hemorrhage, do an MRA of the head and neck. Some institutions may substitute computed tomography angiography (CTA) or other vascular imaging.
If the history is suspicious for arterial dissection or if no other cause is found, perform MRI of the neck with fat saturated images to best visualize the lumen of the artery.
If imaging or history point to SVT, and the imaging above has not been sufficient to visualize the veins, consider additional dedicated imaging with MR or CT venography or the venous phase of conventional angiography.
Vascular Causes
The most well-known causes of thunderclap headache are subarachnoid hemorrhage and unruptured intracranial aneurysm. However, if the patient has focal neurologic deficits the differential should include ischemic stroke, intraparenchymal hemorrhage, SVT, and cervical artery dissection. Blood near the circle of Willis points to hemorrhage from aneurysm whereas blood in the cortical convexities has been associated with SVT, reversible cerebral vasoconstriction, PRES, and cavernomas (42). Chapters 10, 11 and 12 discuss subarachnoid hemorrhage, aneurysm, intraparenchymal hemorrhage, ischemic stroke, arterial dissection, and SVT.
Reversible Cerebral Vasoconstriction Syndrome
The syndrome now called RCVS was previously given several other labels by different medical subspecialists including Call–Fleming syndrome, benign angiopathy of the CNS, and postpartum angiopathy. The current descriptive term highlights the major features of the condition—there is multifocal segmental constriction of intracranial vessels which must by definition resolve within 12 weeks. Vasoconstriction may not be demonstrated on initial imaging, so in patients with recurrent thunderclap headache repeated vascular imaging is recommended. The syndrome is more common in women, but the 4 reported pediatric cases have been in boys (64,65).
RCVS has been associated with the postpartum period and a number of drugs including cannabis, selective serotonin reuptake inhibitors, nasal decongestants, ecstasy 3,4-methylenedioxy-N-methamphetamine (MDMA), cocaine, amphetamines, alcohol, nicotine patches, ergot derivatives, triptans, tacrolimus, cyclophosphamide, erythropoietin, intravenous immune globulin, interferon-A (42), licorice (66), and oral contraceptive pills (67). There have been a few reported cases of RCVS in the setting of catecholamine-secreting tumors including pheochromocytoma, or triggered by orgasm or immersion in a hot bath (42). One of the pediatric cases occurred following a deep dive into a swimming pool (64).
Complications are quite serious and time-dependent. Cortical subarachnoid and intraparenchymal hemorrhage, PRES, and seizures tend to occur within 1 week of onset, while stroke and transient ischemic accidents tend to occur 1 to 2 weeks after the initial headache (68). Treatment involves removal of the inciting substance when possible. Calcium channel blockers, most commonly nimodipine, have been used to prevent vasospasm, and intravenous magnesium is often used in postpartum cases (42). The optimum duration of these treatments is unknown. Mild headache often persists, and depressed mood can be seen a few months later (68).
Pituitary Apoplexy
Pituitary apoplexy refers to the sudden onset of severe headache, vision loss, ophthalmoplegia, vomiting, pituitary failure, and sometimes altered consciousness or fever related to hemorrhage or infarction of a pituitary adenoma. Symptoms arise because sudden increase in the contents of the sella leads to compression and infarction of the pituitary gland, and puts pressure on the optic nerves/chiasm and cranial nerves of the cavernous sinus. Leakage of blood or necrotic tissue causes chemical meningitis and headache. While the injury almost always arises from an adenoma, the tumor can be nonsecreting, and in 60% of patients there is no preceding sign of illness. Trauma, hypotension or hypertension, history of radiation therapy, increased ICP, cardiac surgery, treatment with anticoagulation or dopamine agonists, and pituitary function testing have been reported as precipitants but usually the problem arises without clear provocation (69). Hemorrhage into or hemorrhagic infarction of a Rathke cleft cyst can present similarly, and can only be differentiated pathologically (70). Physical examination may reveal vital sign abnormalities indicating pituitary dysfunction, vision loss, eye movement abnormalities, and alteration in consciousness. CT and MRI will show a sellar mass, usually with hemorrhage. The differential diagnosis includes subarachnoid hemorrhage from ruptured anterior communicating artery aneurysm, and pituitary adenomas and cerebral aneurysms have a co-occurrence rate of ∼7%, so vascular imaging may be needed. LP does not aid the diagnosis; CSF studies may be normal or may show hemorrhage or meningitis. Initial management should be focused on fluid and electrolyte balance and replacement of pituitary hormones. Many patients require surgical decompression of the adenoma (69,71). Rare cases in children as young as 6 years old have been reported (72).
Colloid Cyst
Colloid cysts are rare intracranial tumors, usually presenting in the fourth or fifth decade of life but have been found in children as young as 3 weeks of age. They are typically located in the anterior third ventricle, leading to obstruction of flow through the foramen of Monro and compression of the fornix. Bilateral frontal or diffuse headache is the most common presenting sign, caused by acute hydrocephalus. Typically the headache begins abruptly, lasts seconds to hours, and may improve with changing head position or lying down. Other symptoms include nausea, vomiting, alteration in personality, memory disturbance, blurred vision, leg weakness, ataxia, and problems with urination. An infrequent but diagnostic association is abrupt loss of consciousness at the peak of the headache. Sudden death has been reported in up to 10% of patients with colloid cyst, sometimes after a long period of symptoms (73). Airplane travel and head trauma have also been reported to precipitate sudden death (74). Overall, children with colloid cyst tend to have a more aggressive course, and present with signs or symptoms of increased ICP (75,76).
Physical exam may show papilledema, cerebellar abnormalities, nystagmus, and Babinski sign. Imaging is diagnostic. The cyst is usually hyperdense but can be hypodense or isodense on CT and may enhance with contrast. MR characteristics are similarly variable, but most frequently the lesion is hyperintense on T1-weighted images and hypointense on T2-weighted images. The differential diagnosis of a mass in the anterior third ventricle includes ependymoma, glioma, craniopharyngioma, choroid plexus papilloma, meningioma, pituitary adenoma, basilar artery aneurysm, or arteriovenous malformation. LP should be avoided if colloid cyst is suspected because it can provoke herniation. Because of the high rate of death, even in the absence of hydrocephalus at the time of imaging, all symptomatic colloid cysts should be removed surgically, and ventricular drainage may be necessary to relieve hydrocephalus. There is disagreement about whether incidentally found colloid cysts should be removed (73,74).
Occipital Location
This is a risk factor for serious secondary cause of headache (77).
Cervicogenic Headache
Cervicogenic headache refers to a head or facial pain that is referred from the neck. This type of headache should be considered in a patient with a history of neck trauma or surgery to the head, or if the headaches occur with movement or palpation of the neck. If this type of headache is suspected, consider imaging of the brain and cervical spine to exclude any acute fractures or injury to the cord. If no acute abnormality is found, physical therapy may be beneficial to the patient (13). For patients with trisomy 21, the American Academy of Pediatrics recommends careful neurologic evaluation for signs and symptoms consistent with spinal cord injury, including loss of bowel or bladder control, neck pain, neck stiffness, as the most important clinical predictor of symptomatic atlantoaxial instability and dislocation (78). Patients with rheumatoid arthritis are also at risk for cervicogenic headache. It is important to remember that many of these patients may localize and actually describe their neck pain as headaches.
Pattern: Progressive or New
Headache Associated With Trauma
Mild head trauma is one of the most common causes of headache presenting to the pediatric emergency department (77). Posttraumatic headache is more common after mild injury and in patients with a prior history of a headache. By definition, the pain must start within 7 days of injury; it usually resolves within 4 weeks of injury, but can become chronic. The headache usually resembles tension-type headache or migraine without aura and may be accompanied by neuropsychological symptoms such as mood changes, reduced attention span, easy distractibility, decreased concentration, and sleep disorders. Whiplash injury can also cause headache (79).
Very rarely, trivial head trauma provokes episodes of hemiplegic migraine, diffuse brain edema, and early seizures. This has been linked to a mutation in the CACNA1A gene in 5 patients (80,81). Another rare effect is the “second impact syndrome,” where repeated mild head trauma leads to diffuse brain edema (82). Worrisome problems like hemorrhage, carotid or vertebral artery dissection, brain edema, and seizures are more commonly the result of severe head trauma. Patients should be treated aggressively for these sequelae, and the headache should be managed symptomatically with analgesic medications. Acetaminophen and limited narcotics may be given acutely.
Nonsteroidal antiinflammatory drugs (NSAIDs) should be avoided for the first few days unless hemorrhage has been excluded. Chronic posttraumatic headache should be managed like the headache it resembles, focusing on a long-term strategy and avoiding overuse of analgesics.
Brain Tumor
When persistent headaches are present in children, families are often worried about the possibility of an underlying primary brain tumor. Characteristics that predict the presence of a space-occupying mass in children with recurrent headaches include headache lasting less than 1 month, the absence of a family history of migraine, an abnormal neurologic exam, gait abnormalities, and the occurrence of seizures. Other risk factors for brain tumor include sleep-related headache, vomiting, and confusion (83). Brain tumors should also be considered if a child has a history of exposure to ionizing radiation or an underlying syndrome such as tuberous sclerosis or neurofibromatosis (13).
If a patient is found to have a brain tumor, potential etiologies of headache include hydrocephalus, mass effect due to vasogenic edema, hemorrhage, and secondary metabolic disturbances, such as changes in serum sodium. For example, hypernatremia in the setting of diabetes insipidus can occur in the setting of both primary or secondary brain tumors as well as infiltrative diseases such as Langerhans cell histiocytosis (84). Alternatively, hyponatremia in the setting of cerebral salt wasting or syndrome of inappropriate antidiuretic hormone (SIADH) can also occur if a tumor is present.
While treating headaches associated with primary tumors can be challenging, choosing a treatment often depends on the underlying cause of the headache. For instance, if increased ICP is a leading cause, consider the use of acetazolamide. If the tumor is exerting mass effect, treatment with steroids may be helpful to reduce the edema. If awaiting definitive treatment with surgery, using narcotics as a bridge may be appropriate.
Pattern: Parents—Lack of Family History
This is a nonspecific risk factor for secondary cause of headache, including structural causes like brain tumor. Most patients with a primary headache disorder have a family history of headache. However, because primary headache disorders are so much more common than serious secondary causes of headache, most patients without a family history of headache will still have a primary headache disorder like migraine.
Pattern: Precipitated by Valsalva
Most headaches are briefly worsened by coughing, sneezing, and straining, so it is concerning only if the headache worsening is severe and/or sustained. Valsalva maneuvers can also lead to spontaneous CSF leak with resultant low pressure headache.
Chiari Malformation
Chiari malformation type I is defined as inferior displacement of the cerebellar tonsils at least 5 mm below the foramen magnum. It is detected on about 1% of MRIs in children, often incidentally when imaging is done for unrelated reasons. The only population-based study of children, extracted from the database of the Kaiser system, showed that 63% with this finding were symptomatic, including 55% with headache (85). Other associated symptoms include neck pain, syringomyelia (fluid in the center of the spinal cord), motor problems, scoliosis, dysphagia, ataxia, and rarely syncope. The headache can be nonspecific, but the classic presentation is short-lasting stabbing occipital pain precipitated by cough or Valsalva maneuvers like sneezing, laughing, straining, lifting, or bending over (86).
The relationship between the malformation and headache is not fully understood—since Chiari malformations are relatively common (1%) and headaches are very common, it is not always clear that the malformation is causing the headache. The question then arises whether these patients would benefit from surgery. Most of the information in the literature comes from case series of children who have had posterior fossa decompression surgery to relieve the symptoms. There is some disagreement among neurosurgeons about the appropriate criteria for surgery. In their series of 500 children who had surgery for Chiari I, Tubbs et al (87) described that > 80% of patients with Valsalva-induced headache/neck pain improved, but only 15% of those with non–Valsalva-induced headaches improved. Small case series of children under 6 years old have reported more favorable outcomes, with 76% to 100% improvement in headache, including Valsalva-induced and non–Valsalva-induced (88–90). McGirt et al (91) conducted a review of their 256 pediatric patients who had decompressive Chiari surgery to look at long-term outcomes and found that headache is more likely to recur after surgery than are other preoperative symptoms, and the risk is increased for frontal headaches and headaches of longer preoperative duration. Whereas all other studies were conducted from neurosurgical case series, the population-based Kaiser study reported that three of the 28 children who presented with headache had surgical decompression for intractable daily headache. All three improved postoperatively, but one had recurrence of the headache soon after surgery (85). Current research is using advanced imaging studies to look for factors that could predict who will benefit from surgery (92).
Pattern: Positional
When eliciting a history of headache worsened by change in position, it is imperative to clarify the conditions in which the headache is exacerbated.
Worse when upright: Migraine headaches worsen with movement, and therefore are usually worse when the patient is upright and better when the patient is lying down. However, this is different from the dramatic change expected with headache from IH soon after the CSF leak, where the headache should essentially disappear when the patient lies down for at least 15 minutes, and come back only when the patient gets up. This dramatic change can disappear over time, so ask about whether there was an effect of change in position early in the course of the headache. Postural orthostatic tachycardia syndrome (POTS) will also cause headache which is worse when upright and active.
Worse when lying down: This is a sign of increased CSF pressure. Patients will often report that they are more comfortable upright, or that they are awakened by headache in the middle of the night after lying down for several hours. While migraine and other primary headaches can come at night, this history should always raise concern for structural lesions that cause increased CSF pressure.
Worse with neck movements: Cervicogenic headaches and headaches related to trauma will worsen with neck movements. Many patients with migraine also complain of neck pain.
Intracranial Hypotension
Intracranial hypotension (IH) is a condition where the leak of CSF through the dura leads to low CSF volume and/or pressure. IH can develop spontaneously or from minor trauma, LP, epidural or spinal anesthesia, or surgery. All of these causes have been described in children (93,94).
Typically the presentation is insidious, with diffuse, dull pain which worsens when upright for 15 minutes, and improves when supine, but headache from spontaneous CSF leak can start with thunderclap onset. Over time the effect of position on the headache lessens. Muffled hearing, hyperacusis, tinnitus, blurred vision, nausea, neck pain, and stiffness often accompany the headache. When related to a procedure the pain usually starts within 24 to 48 hours, but can be as late as several weeks afterwards (95). The rate of post-LP headache in pediatric studies has varied between 4% and 50%, lower in children ≤ 12 years and with higher gauge (25 or 27) needles (94). Connective tissue diseases such as Ehlers-Danlos and Marfan syndrome have been associated with spontaneous leak. The presence of CSF rhinorrhea suggests leak at the cribriform plate, but most spontaneous leaks occur in the thoracic spine.
Brain MRI with gadolinium usually shows diffuse pachymeningeal enhancement, downward displacement of the brain (can mimic Chiari malformation), enlargement of the pituitary (can mimic pituitary tumors), and engorgement of the venous sinuses sometimes with subdural hygromas or hematomas. Rarely the brain MRI is normal and nuclear cisternogram or CT or MR myelogram must be pursued to document the leak.
Conservative treatment involves supine positioning, intravenous hydration, and intravenous caffeine. Often lumbar blood patch brings relief (96) (even if the leak is not in the lumbar area), but sometimes blood patch directed to the area of leak or even surgical repair are needed (42).
Postural Orthostatic Tachycardia Syndrome
POTS is a form of orthostatic intolerance that is thought to be secondary to autonomic dysfunction. While typically characterized by a constellation of symptoms including light-headedness, syncope, nausea, palpitations, and visual changes, headache is one of the most common symptoms. In terms of epidemiology, postpubescent adolescent females are generally most affected and its development has been commonly reported after an underlying trigger such as a prolonged illnesses or injury. Although diagnostic guidelines are not definitive, it has been suggested that an increase of more than 35 beats per minute with standing (without an obvious underlying cause) in the context of worsening clinical symptoms is consistent with this diagnosis (97). In terms of headache, patients typically report orthostatic headaches, ones that are worsened with standing and improved when recumbent and often occipital in location or affecting the nape of the neck. Additionally, migraines are often comorbid with POTS and it has been reported that up of 95% of patients affected with POTS have concurrent nonorthostatic headaches meeting the definition of migraine or probable migraine.
Years <3
This is a nonspecific risk factor for secondary headache, driven in part by the fact that it is difficult to get a full history and physical examination from a very young child. In their review of patients presenting to the emergency department for headache, Conicella et al (77) found age 2 to 5 years to be a risk factor for life-threatening secondary cause of headache. Because there is little data from which to derive a recommendation, the lower age cutoff of 3 years here is based on expert opinion (98; D. Lewis, A. Hershey, and M. Yonker, personal oral communication, April 14–15, 2011). While very young children can suffer from primary headache disorders like migraine, at initial presentation the clinician should look for signs and symptoms of secondary causes of headache like infection and tumor. In general for this age group, consider obtaining additional diagnostic tests like brain MRI.
■ PRIMARY HEADACHE DISORDERS THAT MIMIC DANGEROUS HEADACHES
In an emergent and critical care setting, it is important to be able to identify mimics of dangerous headaches. In fact, there are many primary headache disorders in children that have features that may resemble more serious neurologic conditions such as stroke, increased ICP, and shunt malfunctions. In addition, it also important to remember that a child may have more than one cause of headache. For instance, while children with shunts are at risk for headaches secondary to shunt malfunction and infection, they may also experience migraines. As a result, it is important to keep these primary headache disorders in the differential when a patient presents with headache and other paroxysmal neurologic signs or symptoms.
In one retrospective study that looked that the frequency and characteristics of headaches that presented to an emergency room in 1 year, 38% of patients presented with a headache secondary to a primary headache disorder. Migraine (especially migraine without aura) was most common followed by tension headache, chronic migraine, and cluster headache (77). Headaches attributed to a serious life-threatening intracranial disorder occurred in about 6% of patients; these etiologies included brain tumors, meningitis, ventriculoperitoneal shunt malfunction, IIH, and brain malformations (Dandy-Walker, Chiari malformations). In this study, all patients with primary headaches were able to describe the quality of their pain while the majority of patients with intracranial diseases were unable to do so. In addition, primary headaches represented about 70% of the unilateral headaches while those with headaches attributed to intracranial disease were either unable to locate their pain or described an occipital headache (77).
The need for additional diagnostic workup for a patient with headache depends on the quality, pattern, and associated symptoms that are identified. If a patient has recurrent paroxysmal headaches that are typical of migraines with a normal neurologic exam and a family history of migraine, typically no imaging or further workup is required (83). However, if a patient with a history of recurrent migraine with a previously normal exam presents with an atypical headache or is found to have an abnormal neurologic exam, additional testing should be considered to rule out other organic causes (99). Other elements in a patient’s history that may prompt further diagnostic investigations include recent head or neck trauma, new onset acute headache, new symptoms of increased ICP, worsening severity, and systemic symptoms such as fever, weight loss, and nuchal rigidity (99,100).
Migraine can cause many different focal abnormalities. Migraine with aura, basilar-type migraine, and hemiplegic migraine may be mistaken for stroke at initial presentation. Migraine equivalents also include paroxysmal events without headache like cyclic vomiting, abdominal migraine, benign paroxysmal vertigo, and benign paroxysmal torticollis (101). Common migraine auras include visual, sensory, and speech disturbances. Patients with a history of migraine with aura who present with typical aura (defined as less than 60 minutes, but most important is that the timing be within the patient’s typical range) do not require imaging, even if the aura is not followed by headache. Those with prolonged aura should be evaluated because these patients have an increased risk of ischemic stroke. Basilar-type migraine often presents with vertigo, visual disturbances, bilateral sensory symptoms, and ataxia. Should these symptoms present for the first time, a full workup must be done to exclude other disorders involving the posterior fossa.
Hemiplegic migraine is described as migrainous headache with an associated aura that produces fully reversible motor weakness. The weakness will often precede the headache, has been reported to persist even after resolution of the headache itself, and is more likely to affect the arm than the leg (102). Typically the headache is located contralateral to the focal deficit. Hemiplegic migraine is often familial, with at least 1 first- or second-degree relative being affected and 3 types have been described. Type 1 is an autosomal dominant disorder associated with mutation in the CACNA1A gene. Type 2 is associated with a mutation in the ATP1A2 gene (alpha2 subunit of the sodium potassium pump) and type 3 is associated with a mutation in the SCN1A gene. Sporadic hemiplegic migraine has also been described in patients with clinical episodes of hemiplegic migraine and no family history (103). Though no cases have been reported in children, a case series of adults described a condition called Migraine with Unilateral Motor Symptoms (MUMS) characterized by unilateral “give-way” weakness of arm and leg typically ipsilateral to the headache; the weakness could be prolonged, and was seen in patients with both episodic and chronic migraine (104). In terms of treatment, case reports have found verapamil and acetazolamide to be effective for hemiplegic migraine. Triptans are typically contraindicated (105).
Other primary headache disorders seen rarely in children include cluster headaches and paroxysmal hemicranias. Cluster headaches are severe stabbing headaches localized to the periorbital or temporal regions that are unilateral and associated with autonomic phenomenon such as rhinorrhea, lacrimation, and conjunctival injection. This headache syndrome is generally characterized by attacks of daily headaches lasting 15 minutes to 3 hours. These attacks can be ongoing for weeks and then remit for weeks to months at a time with the patient being headache free. Paroxysmal hemicrania is similar to cluster headaches in character though headaches are shorter (few minutes) and occur more frequently throughout the day during an attack than cluster headaches (21). Paroxysmal hemicrania is generally responsive to indomethacin while cluster headaches are not. If a patient presents with these particular headaches, it is important to get brain imaging as they can occur in the setting of pituitary or vascular abnormalities.
If other conditions have been ruled out by history or diagnostic tests and the diagnosis of migraine is most appropriate, acute treatment could include a wide variety of medications like NSAIDs, antidopaminergic medications, dihydroergotamine, and valproic acid (106,107). The best studied combination in children is prochlorperazine and ketorolac (108). Steroids and intravenous magnesium have not been studied in children but are also used. In critically ill patients, it is especially important to consider the side effects of many of the medications used. For example (this list is not exhaustive), NSAIDs and steroids can cause gastrointestinal upset and hypertension. Antidopaminergic medications like metoclopramide and prochlorperazine can cause restlessness or dystonic reaction. Dihydroergotamine is an alpha-adrenergic blocker and a weaker arterial vasoconstrictor; it is contraindicated in patients with hypertension, ischemic heart disease, and residual cardiac abnormalities from Kawasaki disease because it can cause sustained coronary artery constriction. Valproic acid is teratogenic, so a pregnancy test should be done on a patient before it is used. Acute migraine management has been reviewed recently (109).
■ CONCLUSIONS
Though headaches are very common, and a high proportion of children experience a severe headache at some time, there are many serious causes of headache. Headaches can be a symptom of a primary headache disorder like migraine, but even these can have unusual features. Headaches can also be secondary to another underlying problem. A thorough history and physical should guide the evaluation of headache to ensure that these serious causes are detected when present.
■ ACKNOWLEDGMENT
Special thanks to Dr William Young for discussion of the differential diagnosis of severe headaches.
■ REFERENCES
1. . Pearls: headache. Semin Neurol. 2010;30(1):74–81.
2. et al. Headache etiology in a pediatric emergency department. Pediatr Emerg Care. 1997;13(1):1–4.
3. , , . Headaches in a pediatric emergency department: etiology, imaging, and treatment. Headache. 2000;40(1):25–29.
4. , . Headaches attributable to infectious diseases. Curr Pain Headache Rep. 2010;14(4):299–308.
5. , , . Mumps. Lancet. 2008;371(9616):932–944.
6. et al. Tuberculosis of the central nervous system in children: a 20-year survey. J Infect. 2000;41(1):61–68.
7. . New daily persistent headache in children and adults. Curr Pain Headache Rep. 2009;13(1):47–51.
8. et al. An otolaryngology, neurology, allergy, and primary care consensus on diagnosis and treatment of sinus headache. Otolaryngol Head Neck Surg. 2006;134(3):516–523.
9. et al. Sinusitis in children and adolescents with chronic or recurrent headache: a case-control study. J Headache Pain. 2008;9(1):33–36.
10. , . Acute isolated sphenoid sinusitis. Ann Acad Med Singapore. 2004;33(5):656–659.
11. et al. Acute isolated sphenoid sinusitis in children. Pediatr Infect Dis J. 1997;16(12):1180–1182.
12. , . Secondary headaches in children and adolescents. In: , , , eds. Headache in Children and Adolescents. BC Decker Inc., Hamilton, Ontario, PMPHUSA; 2007.
13. , . Secondary causes of headaches in children: when it isn’t a migraine. Pediatr Ann. 2010;39(7):431–439.
14. et al. Acute disseminated encephalomyelitis: a review of 18 cases in childhood. J Paediatr Child Health. 2003;39(5):336–342.
15. , . The metabolic headaches. Curr Pain Headache Rep. 2008;12(4):292–295.
16. . Headache. In: , , eds. Netter Pediatrics. Philadelphia, PA: Elsevier Saunders; 2011:471–478.
17. , , . Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150–1159.
18. , . Central nervous system involvement in pediatric rheumatic diseases: current concepts in treatment. Curr Pharm Des. 2008;14(13):1295–1301.
19. , , . Late postpartum eclampsia in adolescents. Pediatr Emerg Care. 2007;23(6):401–403.
20. et al. Headache as the only neurological sign of cerebral venous thrombosis: a series of 17 cases. J Neurol Neurosurg Psychiatry. 2005;76(8):1084–1087.
21. Headache Classification Subcommittee of the International Headache Society. International Classification of Headache Disorders. 2nd ed. Cephalgia. 2004; 24 Suppl 1:1–160
22. , . Postictal headache. Epilepsy Behav. 2010;19(2):151–155.
23. et al. Headache associated with epileptic seizures: epidemiology and clinical characteristics. Headache. 2002;42(7):649–655.
24. . Characteristics of migraine visual aura. Paper presented at: American Headache Society 53rd Annual Scientific Meeting; 2011; Washington, DC.
25. et al. Migralepsy: a call for a revision of the definition. Epilepsia. 2009;50(11):2487–2496.
26. . Late onset childhood occipital epilepsy. International League Against Epilepsy Web site. http://www.ilae-epilepsy.org/ctf/syn_frame.html. Updated October 3, 2003. Accessed March 4, 2011.
27. . Early onset benign childhood seizures with occipital spikes (Panayiotopoulos syndrome). International League Against Epilepsy Web site. http://www.ilae-epilepsy.org/ctf/syn_frame.html. Updated August 20, 2003. Accessed March 4, 2011.
28. . Panayiotopoulos syndrome: a benign childhood autonomic epilepsy frequently imitating encephalitis, syncope, migraine, sleep disorder, or gastroenteritis. Pediatrics. 2006;118(4):e1237–e1243.
29. et al. Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia. 2006;26(4):361–372.
30. et al. Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia. 2009;29(7):719–728.
31. et al. Clinical manifestations in children with mitochondrial diseases. Pediatr Neurol. 2010;43(3):183–189.
32. . Headache and mitochondrial disorders. Headache. 2008;48(5):733–734.
33. , . Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes (MELAS). GeneReviews. Oct 2010. PMID: 20301411.
34. , , . MELAS presenting as migraine complicated by stroke: case report. Neuroradiology. 1997;39(11):781–784.
35. , , . POLG-Related Disorders. GeneReviews. March 2010. PMID: 20301791.
36. et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy resulting in stroke in an 11-year-old male. Dev Med Child Neurol. 2009;51(9):754–757.
37. et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol. 1998;44(5):731–739.
38. et al. Apathy: a major symptom in CADASIL. Neurology. 2009;72(10):905–910.
39. et al. Genetically confirmed CADASIL in a pediatric patient. Pediatrics. 2010;126(6):e1603–e1607.
40. , , . Hypertensive encephalopathy, reversible occipitoparietal encephalopathy, or reversible posterior leukoencephalopathy: three names for an old syndrome. J Child Neurol. 1999;14(5):277–281.
41. , . Posterior reversible encephalopathy. J Intensive Care Med. 2012 Feb;27(1):11–24.
42. , . Abrupt-onset severe headaches. Semin Neurol. 2010;30(2):192–200.
43. et al. Clinical experience of childhood hypertensive encephalopathy over an eight year period. Chang Gung Med J. 2008;31(2):153–158.
44. , ., . Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol. 2001;24(5):361–364.
45. et al. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205–210.
46. et al. Syndrome of transient headache and neurological deficits with CSF lymphocytosis (HaNDL) associated with recent human herpesvirus-6 infection. Cephalalgia. 2009;29(4):487–491.
47. et al. Usefulness of multimodal MR imaging in the differential diagnosis of HaNDL and acute ischemic stroke. BMC Neurol. 2010;10:120.
48. , . Pseudomigraine with lymphocytic pleocytosis. Curr Pain Headache Rep. 2003;7(3):224–228.
49. . Idiopathic intracranial hypertension (pseudotumor cerebri). Curr Neurol Neurosci Rep. 2008;8(2):87–93.
50. , . Advances in evaluation and management of pediatric idiopathic intracranial hypertension. Curr Opin Ophthalmol. 2008;19(5):391–397.
51. et al. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med. 2010;363(9):891–893.
52. et al. Utility of CSF pressure monitoring to identify idiopathic intracranial hypertension without papilledema in patients with chronic daily headache. Cephalalgia. 2004;24(6):495–502.
53. et al. Abnormal pressure waves in headache sufferers with bilateral transverse sinus stenosis. Cephalalgia. 2010;30(12):1419–1425.
54. , . Recurrent pineal apoplexy in a child. Neurology. 2007;69(1):112–114.
55. et al. Pineal cyst apoplexy: case report and review of the literature. Neurosurgery. 2005;57(5):E1066; discussion E1066.
56. , . Arachnoid cysts: case series and review of the literature. Neurosurg Focus. 2007;22(2):E7.
57. et al. Spontaneous reduction of intracranial arachnoid cysts: a complete review. Br J Neurosurg. 2008;22(5):626–629.
58. , . A population-based study of intracranial arachnoid cysts: clinical and neuroimaging outcomes following surgical cyst decompression in children. J Neurosurg. 2006;105(5 suppl):385–390.
59. et al. Arachnoid cysts: does surgery improve epileptic seizures and headaches? Neurosurg Rev. 1995;18(3):173–181.
60. et al. Failure of cerebrospinal fluid shunts: part I: Obstruction and mechanical failure. Pediatr Neurol. 2006;34(2):83–92.
61. et al. Failure of cerebrospinal fluid shunts: part II: overdrainage, loculation, and abdominal complications. Pediatr Neurol. 2006;34(3):171–176.
62. . Shunt-related headaches: the slit ventricle syndromes. Childs Nerv Syst. 2008;24(4)423–430.
63. . and , Personal Communication. 2010.
64. et al. A pediatric case of reversible segmental cerebral vasoconstriction. Can J Neurol Sci. 2006;33(2):250–253.
65. et al. Three paediatric patients with reversible cerebral vasoconstriction syndromes. Cephalalgia. 2010;30(3):354–359.
66. et al. Licorice-associated reversible cerebral vasoconstriction with PRES. Neurology. 2010;75(21):1939–1941.
67. et al. Reversible cerebral vasoconstriction syndrome with posterior leucoencephalopathy after oral contraceptive pills. Cephalalgia. 2010;30(1):42–45.
68. et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130(pt 12):3091–3101.
69. et al. Pituitary tumor apoplexy: a review. J Intensive Care Med. 2008;23(2):75–90.
70. et al. Clinicopathological characteristics in patients presenting with acute onset of symptoms caused by Rathke’s cleft cysts. Acta Neurochir (Wien). 2010;152(10):1673–1678.
71. . et al. Pituitary apoplexy: an overview of 186 cases published during the last century. Acta Neurochir (Wien). 2010;152(5):749–761.
72. et al. Pituitary apoplexy in a child. Case report. J Neurosurg. 1973;39(4):519–522.
73. . Colloid cyst headache. Curr Pain Headache Rep. 2004;8(4):297–300.
74. , , . Colloid cyst: a case report and literature review of a rare but deadly condition. J Emerg Med. 2011;40(1):e5–e9.
75. et al. Pediatric colloid cysts of the third ventricle: management considerations. Acta Neurochir (Wien). 2010;152(3):451–461.
76. , . Colloid cysts in children, a clinical and radiological study. Childs Nerv Syst. 2006;22(5):514–516.
77. et al. The child with headache in a pediatric emergency department. Headache. 2008;48(7):1005–1011.
78. . Health supervision for children with Down syndrome. Pediatrics. 2011;128(2):393–406.
79. , , . Post-traumatic headache: is it for real? Crossfire debates on headache: pro. Headache. 2010;50(4):710–715.
80. et al. Early seizures and cerebral oedema after trivial head trauma associated with the CACNA1A S218L mutation. J Neurol Neurosurg Psychiatry. 2009;80(10):1125–1129.
81. et al. Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol. 2001;49(6):753–760.
82. , , . Second impact syndrome: concussion and second injury brain complications. J Am Coll Surg. 2010;211(4):553–557.
83. et al. Practice parameter: evaluation of children and adolescents with recurrent headaches: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;59(4):490–498.
84. et al. Central nervous system disease in Langerhans cell histiocytosis. J Pediatr. 2010;156(6):873–881, 881 e1.
85. et al. Chiari type I malformation in a pediatric population. Pediatr Neurol. 2009;40(6):449–454.
86. . Teaching case: Chiari type I/ cerebellar ectopia headaches: complete resolution following posterior fossa decompressive surgery. Headache. 2008;48(7):1146–1149.
87. et al. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation Type I. J Neurosurg Pediatr. 2011;7(3):248–256.
88. et al. Chiari malformation Type I in children younger than age 6 years: presentation and surgical outcome. J Neurosurg Pediatr. 2010;5(6):554–561.
89. et al. Chiari I malformation in the very young child: the spectrum of presentations and experience in 31 children under age 6 years. Pediatrics. 2002;110(6):1212–1219.
90. et al. Headache and Chiari I malformation in the pediatric population. Pediatr Neurosurg. 1998;29(1):14–18.
91. et al. Symptom recurrence after suboccipital decompression for pediatric Chiari I malformation: analysis of 256 consecutive cases. Childs Nerv Syst. 2008;24(11):1333–1339.
92. et al. Correlation of hindbrain CSF flow and outcome after surgical decompression for pediatric Chiari I malformation. Childs Nerv Syst. 2008;24(7):833–840.
93. , , . Spontaneous intracranial hypotension in childhood: a case report and review of the literature. J Child Neurol. 2011;26(6):761–6.
94. . Dural punctures in children: what should we do? Paediatr Anaesth. 2002;12(6):473–477.
95. et al. Post-dural puncture headaches in children. A literature review. Eur J Pediatr. 2003;162(3):117–121.
96. , . Management of postdural puncture headache with epidural blood patch in children. Paediatr Anaesth. 2002;12(6):526–529.
97. , , . Secondary headache in children. Neurol Sci. 2010;31(suppl 1):S81–S82.
98. . , and , Personal Communication. 2011.
99. , . Common emergent pediatric neurologic problems. Emerg Med Clin North Am. 2002;20(1):155–176.
100. , . When and how to investigate the patient with headache. Semin Neurol. 2010;30(2):131–144.
101. et al. Overview of diagnosis and management of paediatric headache. Part I: diagnosis. J Headache Pain. 2011 Feb;12(1):13–23.
102. et al. Evidence for a separate type of migraine with aura: sporadic hemiplegic migraine. Neurology. 2003;60(4)595–601.
103. . Pediatric migraine. Neurol Clin. 2009;27(2):481–501.
104. et al. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry. 2007;78(6):600–604.
105. , . Child neurology: migraine with aura in children. Neurology. 2010;75(5):e16–e19.
106. , . Management of migraine in children and adolescents in the emergency department and inpatient setting. Curr Pain Headache Rep. 2005;9(5):363–367.
107. , . Emergency department treatment of primary headaches in children and adolescents. Curr Opin Pediatr. 2008;20(3):248–254.
108. et al. Treatment of pediatric migraine headaches: a randomized, double-blind trial of prochlorperazine versus ketorolac. Ann Emerg Med. 2004;43(2):256–262.
109. , . Evaluation and management of children and adolescents presenting with an acute setting. Semin Pediatr Neurol. 2010;17(2):105–108.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


