Placental abruption
Vasa praevia
22
0.5
Trauma
Vulvovaginal varicosities
Genital infections
Genital tumours
Others
5.0
2.0
0.5
0.5
0.5
Diagnosis and Management
Antepartum haemorrhage by nature is unpredictable, and the bleeding at presentation can be significant or non-substantial. The management of any patient with significant APH should ideally be in a hospital with adequate facilities for transfusion, delivery by caesarean section (CS) and neonatal intensive care. Initial management includes history-taking, evaluation of the general condition, initiation of appropriate investigations and treatment including delivery.
History
This must include the amount, character and duration of bleeding. It is also important to ascertain whether there are any associated abdominal pains or regular uterine contractions. Initiating or contributory factors such as trauma or coitus should be excluded. The gestational age as confirmed by either a booking ultrasound scan or the last menstrual period and information regarding placental site should be obtained. Additional useful information includes the number of past bleeding episodes, history of ruptured membranes, past obstetric and cervical smear history.
Physical Examination
This is aimed at assessing both maternal and fetal conditions and includes a general examination for evidence of shock (pallor, restlessness, cold-clammy extremities and poor skin perfusion), assessing maternal pulse, respiratory rate and blood pressure. Abdominal examination includes fundal height measurement, consistency of uterus (soft or firm), presence of tenderness, palpable uterine contractions, fetal lie, presentation and viability. Vulval inspection should include an assessment of the amount of bleeding and determination of whether the bleeding is continuing or not. A speculum examination is essential but should only be done after placenta praevia has been excluded.
Initial Management and Investigations
The initial assessment/resuscitation and investigations is generic for all types of APH, with further treatment tailored according to the severity of bleeding, gestational age of the pregnancy and the cause of bleeding. These should include:
1. Access to intravenous line with one or two wide-bore cannulae (preferably size 14–16 French gauge).
2. Obtaining blood for a full blood count, urea and electrolytes, group and save, and holding of serum for potential cross-matching depending upon the severity of bleeding. In the presence of heavy bleeding, at least four units of blood should be cross-matched. If placental abruption is suspected a coagulation profile should also be checked. Other tests include a Kleihauer Betke test on maternal blood and urine dipstick for protein.
3. Administration of intravenous fluids if bleeding continues or the woman is haemodynamically compromised, while awaiting cross-matched blood. Colloids are the preferred intravenous fluids in such circumstances. Consideration should be given to transfusing O Rhesus (D) negative blood where cross-matching is delayed.
4. An ultrasound scan assessment to confirm placental site once the feto-maternal status is satisfactory. This may not always be necessary.
Subsequent management (conservative or immediate) will depend on the feto-maternal condition and the gestational age of the fetus. These will be discussed under the various types of APH.
Placenta Praevia
Placenta praevia is defined as a placenta sited partially or wholly in the lower uterine segment. If the placenta lies over the cervical os, it is considered as major praevia. Traditionally, different grades have been defined based on the relationship of the placenta to the internal cervical os (Table 13 2). In clinical practice, ultrasound definitions with relation to the cervical os are more commonly used. A placenta that overlaps the cervical os or has its edges less than 20 mm from the os is considered praevia on ultrasound scan.
| Grade | Description |
|---|---|
| I | Placenta is in the lower segment, but the lower edge does not reach the internal os |
| II | Lower edge of the placenta reaches but does not cover the internal os |
| III | Placenta covers the internal os partially |
| IV | Placenta covers the internal os completely |
The prevalence of clinically identified placenta praevia is approximately 4–5/1000 pregnancies [5]. The exact aetiology of placenta praevia is unknown, but it has been shown to be associated with increasing maternal age, parity, smoking, in-vitro fertilization, multiple pregnancies and previous CS. A single CS increases the risk of placenta praevia by 0.65%, three by 2.2% and four or more by 10% [6]. Furthermore, Hershkowitz et al. [7] showed a recurrent risk of 4–8% after one pregnancy was affected by placenta praevia.
Clinical Implication
Placenta praevia can lead to varying degrees of maternal haemorrhage at different gestations, with a significant impact on materno-fetal well-being.
Maternal Risks
These include:
1. Maternal mortality: primarily due to haemorrhage, has reduced from 5% to less than 0.1% since the use of conservative management [8]. In the 2009–12 Confidential Enquiry into Maternal Deaths and Morbidity report, two maternal deaths were reported secondary to placenta praevia [3].
2. Postpartum haemorrhage: this occurs due to inadequate occlusion of the sinuses in the lower uterine segment at the site of the placental bed.
3. Placenta accreta: occurring in approximately 15% of cases with placenta praevia.
4. Air embolism: this is possible if the sinuses in the placental bed are torn.
5. Postpartum sepsis: often secondary to ascending infection.
Fetal Risks
These include:
1. Perinatal mortality, primarily due to prematurity. Previously, the perinatal mortality for cases presenting between 27 and 32 weeks was approximately 20%; however, with conservative management and improved neonatal care this has dropped to 42–81/1000 [9]. In women with placenta praevia the odds ratios for having a preterm delivery, need for neonatal intensive care and low birth weight are 27.7, 3.4 and 7.4 respectively [10].
2. Fetal growth restriction: may occur in approximately 16% of cases and is more likely in women with recurrent bleeding episodes.
3. Major congenital malformations: reports indicate a doubling in women with placenta praevia. The most common are those of the central nervous, cardiovascular, respiratory and gastrointestinal systems.
4. Unexpected fetal death secondary to vasa praevia or severe maternal haemorrhage can occur.
5. Other associated risks are fetal malpresentation, fetal anaemia, umbilical cord prolapse and compression.
Diagnosis
Clinical
Placenta praevia characteristically presents with painless vaginal bleeding. The initial bleed usually occurs in most cases at about 34 weeks and before 36 weeks in more than 50% of cases [11]. In some cases, threatened miscarriage in the second trimester of pregnancy precedes the bleeding due to placenta praevia. The bleeding episodes are not uncommonly recurrent, with the severity of subsequent episodes usually being greater than the previous one.
The absence of abdominal pain is regarded as a significant differentiating feature between placenta praevia and abruption, although 10% of women with placenta praevia will have a co-existing abruption. Since most women undergo a second trimester ultrasound scan and placental localization, low-lying placentae should have been diagnosed. Other findings on abdominal examination include malpresentation of the fetus, which occurs in about 35% of cases [12]. Vaginal examination is avoided in known cases of placenta praevia as speculum or digital examination may further aggravate bleeding. Historically, in cases of suspected placenta praevia with mild to moderate bleeding, where delivery was being considered, a digital vaginal examination was performed in theatre with or without anaesthesia. This so called ‘double set-up examination’ allowed immediate access to CS if the placental edge was felt on examination [13]. With the advent of better imaging modalities this approach is rarely undertaken; however, in the parts of the world where ultrasound is not routinely available this can be useful.
Screening for Low-Lying Placenta
Various radiological methods have been used in the past to localize the placenta, including soft tissue placentography, radioisotope radiography, pelvic angiography and thermography. Currently the gold standard for localizing low-lying placenta is ultrasound scan, with an emerging role for magnetic resonance imaging (MRI). Transabdominal ultrasound scan has a high false-positive rate for detection of low-lying placentae, whereas transvaginal scanning is safe in the presence of placenta praevia and is more accurate. In most obstetric units in the UK, fetal anomaly screening is undertaken between 20 and 24 weeks of pregnancy and includes documentation on placental localization. This examination is used to predict the likelihood of placenta praevia at term. Women with low-lying placentae at 20–24 weeks are offered a repeat scan between 34–36 weeks of gestation to confirm the diagnosis. In cases with asymptomatic suspected major placenta praevia, a transvaginal scan is performed at 32 weeks to confirm the diagnosis (Figure 13.1) and allow planning for third trimester management. A few studies have shown that before 24 weeks’ gestation the placenta can be low-lying in 28% of the scans, but by term only 3% of placentae are low-lying [9]. This is because the placenta ‘migrates’ to the upper uterine segment as the pregnancy advances. The mean rate of placental migration is about 5.4 mm per week. In recent years, ultrasound has been used to predict the likelihood and extent of placental migration and the occurrence of placenta praevia at term. Studies using transvaginal ultrasound have shown that unless the placental edge is reaching the internal cervical os at mid-pregnancy, placenta praevia is unlikely to be present at term [14,15]. Oppenheimer et al. showed that at mid-trimester, if the placental edge overlapped the internal cervical os by >2 cm, placental migration did not occur [16]. When the placental edge was >2 cm away from the internal os, migration always occurred, whereas if the edge was <2 cm from the os (Figure 13.2), placental migration occurred in 88.5% of cases. The significance of the shape of the placental edge to predict placental migration has also been studied. A thick placental edge, defined as thickness of 1 cm or less, within 1 cm from the edge and/or an angle between the basal and chorionic plate of >45° is associated with a higher rate of APH and a lesser chance of placental migration [16,17].
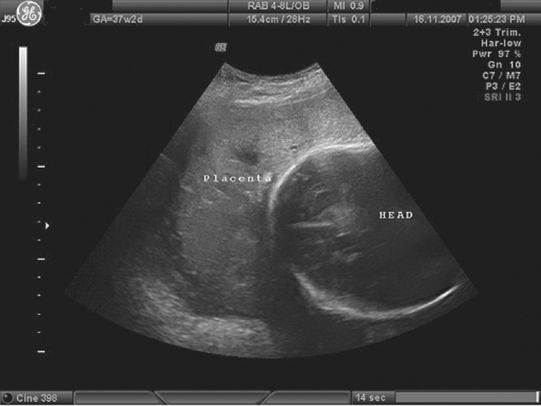
A transvaginal ultrasound image showing a low-lying anterior placenta (placenta praevia) below the fetal head.
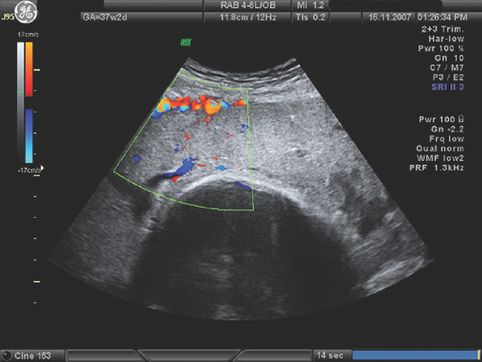
Placenta praevia approximately 18 mm from the cervical os.
A false-negative scan for a low-lying placenta has been reported in 7% of cases. This is primarily seen when the placenta is posterior, the bladder is full, the fetal head obscures the placental margin or the operator fails to scan the lateral uterine wall [18].
Management Options
These are either (a) immediate delivery or (b) expectant management. Both of these are influenced by the severity of haemorrhage, fetal well-being and gestational age.
Immediate Delivery
Where there is severe life-threatening haemorrhage, irrespective of the gestational age, CS is the only delivery option. With mild to moderate bleeding occurring after 34 weeks’ gestation, delivery should be planned after stabilizing the maternal condition.
Expectant Management
In cases where the bleeding is small and self-limiting, expectant management has a role. This provides time to achieve fetal maturity, thereby reducing perinatal morbidity and mortality. Another advantage is that in some cases with advancing gestation, the placenta migrates and vaginal delivery might be considered reasonable. There has been a controversy regarding the expectant management as inpatient or outpatient. Cotton et al. reported no difference in the perinatal or maternal mortality rates in cases managed either at home or in the hospital, whereas others have reported an increase in the neonatal morbidity with those managed at home [9]. For women with asymptomatic placenta praevia, conservative management at home is becoming increasingly acceptable [19]. The RCOG in the UK has recommended that women with major placenta praevia who have previously bled should be admitted and managed as inpatients from 34 weeks of gestation [20]. Those with major praevia who have never bled and are asymptomatic require careful counselling before offering outpatient care. These cases require close proximity with the hospital and constant presence of a companion. During expectant management preterm delivery is a major problem, with approximately 40% occurring before 37 weeks [21]. Papinniemi et al. showed that 88.2% of women with placenta praevia underwent CS before term [10]. Furthermore, the use of tocolysis for uterine contractions with vaginal bleeding is controversial. There is a 10% association of abruption in cases of praevia, and in these the use of tocolysis can mask the features of hypovolaemia. Others have shown reduced perinatal morbidity and mortality with use of tocolysis in preterm labour and placenta praevia [22]. Similarly, the use of cervical cerclage to reduce bleeding and prolong pregnancy is not recommended as sufficient evidence is lacking [23].
Liberal use of blood transfusion has been advocated in cases where there is excessive bleeding. The aim is to optimize oxygen supply to the fetus and restore maternal blood volume, aiming for a haemoglobin of at least 10 g/dl and a haematocrit of 30%. If the bleeding settles, conservative management can be continued on an inpatient basis. Once a significant bleeding episode has occurred, four units of cross-matched blood should be made readily available. Maternal steroids should be administered for fetal lung maturity where indicated. With prolonged inpatient care, mobility and thrombo-prophylaxis should be encouraged and delivery planned around 38 weeks’ gestation.
Mode of Delivery
This is determined by the clinical state of the patient, fetus and the ultrasound findings. Caesarean section is the recommended method for major placenta praevia, whereas vaginal delivery may be possible with minor degrees. Currently, as the diagnosis of praevia is based on ultrasound findings, the distance of the placental edge from the internal os can guide decision making. The RCOG have recommended that the placenta needs to be at least 2 cm from the cervical os for an attempted vaginal delivery [20]. Bhide et al. have suggested that if the placental edge is further than 2 cm from the cervical internal os but within 3.5 cm, vaginal delivery can be attempted [24]. In the UK, the RCOG recommends that for a planned CS for placenta praevia, a consultant obstetrician and anaesthetist should be present within the delivery suite. In the case of an emergency, consultant staff should be alerted and attend as soon as possible. Specialized multidisciplinary personnel like the haematologist and interventional radiologist should be informed and their help sought promptly if required. The American College of Obstetricians and Gynecologists and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists are of the consensus that, when hysterectomy is anticipated, consent should include the same [25,26]. The anaesthetist, in consultation with the obstetrician and the mother, must make the choice of anaesthetic technique for CS. If the patient is not actively bleeding and is in a stable condition, an experienced anaesthetist may consider regional anaesthesia, otherwise general anaesthesia is used. For all cases, whether elective or emergency, cross-matched blood is kept available and the amount depends upon the clinical features of the individual case and availability of the local blood bank services. If the woman has atypical antibodies, specific arrangements for appropriately typed blood should be made with the blood bank. There is no evidence to support the use of autologous blood transfusion in the management of placenta praevia, although cell salvage should be considered where available. The uterine incision in placenta praevia is usually made in the lower segment; however, in difficult cases it may be converted to a T-, J- or U-shaped incision. In the presence of an anterior placenta, the approach can be of either going through the placenta to deliver the baby or identifying the placental edge and going through the membranes above or below the placenta. Some authors advise against cutting or tearing through the placenta as the fetal vessels are torn [27]. Inevitably, the placental bed sinuses bleed as the lower segment is less muscular, with reduced ability for retraction. Where utertonics are not effective, figure-of-eight haemostatic sutures can be applied to the placental bed. Other modalities shown to be effective include intramyometrial prostaglandins, intrauterine hydrostatic balloon and uterine brace sutures. In uncontrolled bleeding, an early decision may be required for uterine or internal iliac artery ligation or even hysterectomy. Embolization of the uterine arteries has been shown to be extremely useful in selective cases. Where the placenta is morbidly adherent it may be left in situ with prophylactic or therapeutic uterine artery embolization and internal iliac artery ligation. The value of methotrexate is debatable. Successful pregnancies have been reported thereafter with a risk of subsequent haemorrhage and need for hysterectomy [28].
Vasa Praevia
Vasa praevia is a rare condition in which the fetal blood vessels traverse the fetal membranes in the lower part of the uterus, unsupported by placental tissue or the umbilical cord. It occurs in 1 per 6000 deliveries [29] and is associated with high perinatal mortality. As the fetal vessels precede the presenting part, they may rupture before or during labour, leading to fetal blood loss. Before the widespread use of ultrasound, vasa praevia was diagnosed retrospectively and the perinatal mortality was high. Characteristic ultrasound features for the diagnosis of vasa praevia include echogenic parallel or circular lines near the cervix representing the umbilical cord, which can be further confirmed by Doppler and transvaginal scan [30,31]. Three-dimensional ultrasound has been shown to be useful in diagnosing vasa praevia [32]. It is important to diagnose these cases antenatally and offer elective CS at term.
Placenta Percreta/Accreta
Placenta accreta or the morbidly adherent placenta occurs due to abnormalities in implantation. It is associated with high maternal morbidity and mortality. In the UK Obstetric Surveillance Study (UKOSS) of women requiring peripartum hysterectomy, 38% had a morbidly adherent placenta, placenta accreta or increta [33]. The prevalence is higher if the placenta is low-lying or there is a prior scar on the uterus. An anterior low-lying placenta with a history of prelabour CS is more likely to be morbidly adherent and for such cases the index of suspicion should be high. Recent reports suggest antenatal diagnosis on ultrasound scan with a high positive predictive value for placenta accreta [34] (Figure 13.3). Three-dimensional colour power Doppler has also been used for diagnosis [35]. One of the specific recommendations of the 2007 CEMACH report is that all women who have had a previous CS should have their placental site determined by ultrasound scan [36]. Magnetic resonance imaging has a poor sensitivity of about 38% and is still considered as a research tool [37]. Management of a morbidly adherent placenta requires multidisciplinary care and planning in the antenatal and intrapartum period.
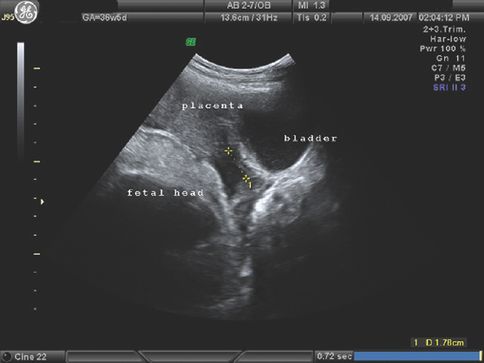
Placenta accreta: note the increased vascularity at the poorly defined placenta–uterine interface.
Placental Abruption
Placental abruption is the most common cause of bleeding in the second and third trimester of pregnancy. It is defined as the partial or complete premature separation of a normally situated placenta. It complicates approximately 0.3–1% of births [38,39], although temporal trends in some countries have shown an increase in the rates of abruption [40,41]. The wide variation in the reported incidence reflects discrepancy in the clinical and histological diagnosis. In one study, histologic evidence of abruption was seen in 4.5% of routinely examined placentae, suggesting that small episodes are more common than the clinical diagnosis [42]. In addition, the incidence of abruption is highest at 24–26 weeks’ gestation, and decreases with advancing gestational age [43].
Placental abruption in 65–80% of cases is ‘revealed’ where the blood tracks between the membranes and the decidua, and escapes into the vagina. In the other 20–35% of cases, abruption is ‘concealed’, and the blood accumulates behind the placenta with no obvious external bleeding. Traditionally, four grades of placental abruption have been described (Table 13.3); the most severe grade is reported in 0.2% of pregnancies.
| Grade | Description |
|---|---|
| 0 | Asymptomatic-small retro-placental clot |
| 1 | External vaginal bleeding present. Uterine tenderness and tetany may be present. No sign of maternal shock or fetal distress |
| 2 | External vaginal bleeding may or may not be present. No signs of maternal shock, but fetal distress is present |
| 3 | External bleeding may or may not be present. Marked uterine tetany, a board-like rigidity on palpation. Persistent abdominal pain, maternal shock and fetal distress are present. Coagulopathy may become evident in 30% of cases |
Risk Factors and Aetiopathogenesis
The exact aetiology of placental abruption is unknown, although haemorrhage at the decidual–placental interface and acute vasospasm of the small blood vessels seems to precede the placental separation. Vascular thrombosis can also lead to decidual necrosis and venous haemorrhage. Recently, reduced expression of RCAS1 placental cell membrane protein has been shown in labours complicated by placental abruption [44].
Direct trauma to the abdomen can cause a shearing force, leading to acute placental separation. This mechanism also explains placental separation with sudden intrauterine decompression, following membrane rupture in cases of polyhydramnios or after the delivery of the first twin. Cocaine and drug abuse cause placental vasoconstriction, leading to abruption. Maternal smoking doubles the risk of abruption, whereas if both parents smoke the risk is increased five-fold [41]. A dose–response relationship has been demonstrated between the number of cigarettes smoked and the risk of placental abruption. In addition, women who stop smoking early in pregnancy have the same risk of placental abruption as women who have never smoked. Other risk factors include bleeding in early pregnancy, an elevated second trimester maternal serum alpha-fetoprotein (ten-fold increased risk of abruption) and second trimester notching of the uterine artery Doppler [45]. Pre-eclampsia is associated with a 2.7-fold increased risk of placental abruption [41], chronic hypertension, pregnancy-induced hypertension, premature rupture of membranes and previous CS are other risk factors. The association between thrombophilias and abruption is controversial; therefore in women with placental abruption without a known cause, thrombophilia screening should be considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


