Geoffrey W. Cundiff
Anorectal dysfunction encompasses a variety of conditions that disrupt normal anorectal function. Such conditions can be subdivided as those that cause defecatory dysfunction and fecal incontinence. Although anorectal dysfunction transcends any individual medical specialty, the pathophysiology, evaluation, and management of conditions relevant to obstetricians/gynecologists are presented in this chapter.
Normal Colorectal Function
Anal continence and defecation are complex physiologic processes that require intact and coordinated neurologic and anatomic function, including colonic absorption and motility, rectal compliance, anorectal sensation, and the multifaceted continence mechanism. An understanding of normal physiology and pathophysiology is essential to the treatment of women with anorectal dysfunction.
Stool Formation and Colonic Transit
The colon plays an important role in absorption and regulation of water and electrolytes. As much as 5 L of water and associated electrolytes can be absorbed in one day. Parasympathetic-mediated peristaltic contraction of colonic smooth muscle transfers fecal material to the rectum. A delay in stool transit at the rectosigmoid region of the colon allows for maximal absorption of water and sodium.
Storage
As stool accumulates in the rectosigmoid, rectal distention triggers a transient decrease in the internal anal sphincter (IAS) tone and an increase in the external anal sphincter (EAS) tone, known as the rectoanal inhibitory reflex. Exposure of the anal canal to fecal matter facilitates sampling, whereby the anal canal and its abundant sensory nerves determine stool consistency (i.e., solid, liquid, or gas). Accommodation occurs as the normally compliant rectal vault relaxes in response to increased volume. This cycle, combined with increased rectal distention, stimulates an urge to defecate. This urge can be voluntarily suppressed through cortical control, resulting in further accommodation and activation of the continence mechanism.
Continence Mechanism
Muscles
The key muscles of the continence mechanism are the puborectalis, IAS, and EAS. The puborectalis muscle originates from the pubic rami at the level of the arcus tendineus levator ani and passes laterally to the vagina and rectum in a U-shaped configuration, creating a sling around the genital hiatus. Contraction of the puborectalis muscle narrows the genital hiatus, developing the near 90-degree anorectal angle. The resting tone of the puborectalis muscle serves as the primary continence mechanism for solid stool. The IAS and EAS are essential for continence of flatus and liquid stool. The internal sphincter maintains most of the resting tone for the sphincter complex through autonomic reflex arcs and is essential for passive continence. Although the external sphincter also maintains constant resting tone, it is ultimately responsible for preventing fecal urgency and stress incontinence associated with sudden increases in intra-abdominal pressure. This function is under both voluntary and involuntary control. The anal cushions act as the final anatomic barrier. They fill with blood, causing occlusion of the anal canal.
Nerves
Many pathologic states disrupt normal function through denervation. The IAS receives its sympathetic supply from L5, which passes through the pelvic plexus via the hypogastric plexus. The parasympathetic supply from S2–4 synapses at the pelvic plexus, where it joins the sympathetic nerves. In addition to the parasympathetic and sympathetic components, the autonomic nervous system of the gut has an enteric nervous system (ENS). The ENS provides local circuitry that can contract or relax the gut muscles, as well as impact absorption and secretion. The autonomic ganglia of the ENS, located in the gut, are interconnected to provide local integration and processing of information. The IAS acts through reflex arcs at the spinal cord without voluntary control. The puborectalis (levator ani) is innervated by branches of the S2–4 sacral roots and does not receive direct innervation from the pudendal nerve (1). The EAS is innervated bilaterally by the pudendal nerve (S2–4) via Alcock’s canal. The pudendal nerve fibers cross over at the level of the spinal cord, allowing preservation of EAS function in the event of unilateral damage. The rich sensory supply from the anal canal travels along the inferior rectal branch of the pudendal nerve.
Evacuation
Initiation of defecation is normally under cortical control. As previously discussed, delivery of stool to the rectum stimulates the rectoanal inhibitory reflex, permitting sampling followed by accommodation. Further rectal distention results in an urge to defecate. Evacuation occurs with voluntary relaxation of the pelvic floor muscles (puborectalis muscle and EAS) in conjunction with increased intra-abdominal and intrarectal pressure from Valsalva. This results in widening of the anorectal angle and shortening of the anal canal, which facilitates emptying. Coordinated peristaltic activity of the rectosigmoid assists evacuation. After this process is complete, the closing reflex is initiated, resulting in contraction of the pelvic floor muscles and activation of the continence mechanism.
Epidemiology
The epidemiology of anorectal dysfunction has been best defined in terms of the incidence and prevalence of fecal incontinence. Few studies have been done to assess the incidence and prevalence of defecatory dysfunction.
Defecatory Dysfunction
The term defecatory dysfunction often is used synonymously with the symptom of constipation. Constipation is an imprecise term used by patients to report a variety of symptoms, including infrequent stools, dyschezia, straining, variation in stool consistency and caliber, incomplete emptying, bloating, and abdominal pain. The most common symptoms associated with constipation are straining and hard stools (2,3). Defecatory dysfunction is defined by many physicians as infrequent stools, typically fewer than three bowel movements per week. This definition is based on stool frequency studies in which 95% of women have more than three bowel movements per week. Using this definition, the prevalence of constipation should be 5% (4). However, the prevalence of constipation has been estimated to range from 2% to 28%, depending on the definition applied (5–7).
There is an increased prevalence of constipation among women and elderly individuals, nonwhite individuals, and those with low income and low education levels (5–7). Based on an estimated 2.5 million visits to US physicians per year for constipation, with an average cost of $2,752 per patient, the annual cost for evaluation of constipation would be approximately $6.9 billion (8,9). An estimated 85% of physician visits results in a prescription; including drug costs would increase this amount substantially (8). More recently, evaluation of 76,854 California Medicaid patients without supplemental insurance found somewhat lower annual total direct costs to care for constipation at almost $19 million ($246 per patient) for this subset of the US population (10). Constipation has a detrimental effect on health-related quality of life (3,10). Constipation contributed to decreased mental and physical scores for quality of life on the SF-36 Health Survey in a Canadian-based population (11).
Fecal Incontinence
The reported prevalence of fecal incontinence varies between 2% and 3% for community-dwelling individuals, 3% to 17% for those of increased age, and 46% to 54% for nursing home residents (12). A prevalence of 28% has been reported among patients seeking benign gynecologic care and 36% of primary care patients surveyed (13,14). The prevalence of fecal incontinence in the United States is expected to increase 59% from 10.6 million in 2010 to 16.8 million in 2050 as the population ages (15). Epidemiologic studies of fecal incontinence are compromised by social stigmata and the lack of a uniform definition. Definitions of fecal incontinence vary with respect to the type of material passed (solid, liquid, or gas), the frequency and duration of events (once in a lifetime to twice a week), and the impact on quality of life. Most authors agree that the true prevalence of this condition is underestimated in the current scientific literature. A large health survey in the United States found age, female gender, physical limitations, and poor general health to be independent risk factors associated with fecal incontinence (16).
Fecal incontinence has tremendous psychosocial and economic implications for individuals and society as a whole. The loss of such a basic function can be emotionally devastating, leading to poor self-esteem, depression, social isolation, and decreased quality of life (13,14,17). Fecal incontinence is the second leading reason for nursing home placement in the United States, even though less than one-third of individuals with this condition seek medical attention (13,17). The overall annual cost to treat fecal incontinence is difficult to pinpoint, but accounts for more than $400 million per year in the cost of adult incontinence products alone (17).
Symptom-Based Approach to Colorectal Disorders
Several medical conditions cause defecatory dysfunction, fecal incontinence, or combined symptoms. Following is the differential diagnosis—a proposed classification system based on systemic factors, anatomic and structural abnormalities, and functional disorders.
Differential Diagnosis
Disordered Defecation
Causes of defecatory dysfunction have traditionally been divided into systemic disorders and idiopathic constipation (all nonsystemic causes). Idiopathic constipation can be subdivided into anatomic and structural abnormalities and functional disorders (Table 28.1).
Table 28.1 Causes of Defecatory Dysfunction and Fecal Incontinence
| Fecal Incontinence | Defecatory Dysfunction | |
| Systemic Factors | ||
| Metabolic/Endocrine | ||
| ● | Diabetes mellitus | ● |
| ● | Thyroid disease | ● |
| Hypercalcemia | ● | |
| Hypokalemia | ● | |
| Neurological | ||
| ● | Central Nervous System | ● |
| Multiple sclerosis, Parkinson disease, stroke, tumor, dementia | ||
| ● | Peripheral Nervous System | ● |
| Hirschsprung disease, spina bifida, autonomic neuropathy, pudendal neuropathy | ||
| Infectious | ||
| ● | Bacterial, viral, parasitic diarrhea | |
| Collagen Vascular/Muscle Disorder | ||
| Systemic sclerosis, amyloidosis, myotonic dystrophy, dermatomyositis | ● | |
| Idiopathic/Autoimmune | ||
| ● | Inflammatory bowel disease | |
| ● | Food allergy | |
| Medications | ||
| ● | Prescription, over the counter | ● |
| Anatomical/Structural Abnormalities | ||
| Pelvic Outlet Obstruction | ||
| ● | Pelvic organ prolapse | ● |
| ● | Descending perineum syndrome | ● |
| Anismus/rectosphincteric dyssynergia | ● | |
| ● | Intussusception, rectal prolapse | ● |
| Volvulus | ● | |
| ● | Neoplasia | ● |
| ● | Benign strictures | ● |
| ● | Hemorrhoids | ● |
| Anal Sphincter Disruption/Fistula | ||
| ● | Obstetrical trauma | |
| ● | Surgical trauma | |
| ● | Anal intercourse | |
| ● | Injury (trauma, radiation proctitis) | |
| Functional | ||
| Motility Disorders | ||
| Global motility disorder | ● | |
| Colonic inertia/slow-transit constipation | ● | |
| ● | Irritable bowel syndrome | ● |
| Functional constipation | ● | |
| ● | Functional diarrhea | |
| Functional Limitations | ||
| ● | Decreased mobility | ● |
| ● | Decreased cognition | ● |
Diabetes, hypothyroidism, and pregnancy are the most common endocrinologic systemic factors that cause constipation, and all have a component of decreased gastrointestinal motility and intestinal transit. In one study, gastrointestinal symptoms were present in 76% of patients with diabetes, including constipation, which occurred in 60% (18). In patients with diabetes, constipation is believed to be secondary to intestinal autonomic neuropathy, resulting in delayed or absent gastrocolic reflex and decreased bowel motility. This enteric neuropathy may also cause gastroparesis and diarrhea. Although diabetes has been classified with the endocrinologic causes, it should also be grouped with the enteric neuropathies. Pregnancy is not considered a disease state; however, there is an 11% to 38% prevalence of constipation that is believed to result from the effect of progesterone on smooth muscle (19,20). Iron supplements and prior constipation treatment are also associated with constipation during pregnancy (20).
The neurologic systemic factors can be divided into central and peripheral processes. Spinal cord lesions, multiple sclerosis, and Parkinson disease affect the autonomic nervous system. Trauma to the sacral nerves often leads to severe constipation from decreased left-sided colonic motility, decreased rectal tone and sensation, and increased distention. These findings are also seen in patients with meningomyelocele, damage to the lumbosacral spine, and pelvic floor trauma (21,22). Higher spinal cord lesions result in delayed sigmoid transit and decreased rectal compliance. In these upper motor neuron lesions, colonic reflexes are intact, and defecation can be initiated by digital stimulation of the anal canal (23,24). Individuals with multiple sclerosis can have no gastrocolic reflex, decreased colonic motility, decreased rectal compliance, and even rectosphincteric dyssynergia (25,26). Constipation worsens with the duration of illness and may be compounded by the side effects of medical therapy. Similar findings of rectosphincteric dyssynergia and medication side effects are present with Parkinson disease.
Among the peripheral neurogenic disorders, dysfunction occurs at the level of the ENS. The ultimate example of this is congenital aganglionosis (Hirschsprung disease). The absence of intramural ganglion cells in the submucosal and myenteric plexuses of the rectum causes loss of the rectosphincteric inhibitory reflex. Patients with this illness usually present with functional obstruction and proximal colonic dilation. In most patients, the condition is diagnosed within 6 months of age, although milder cases can be seen later in life.
Other systemic factors to consider are collagen vascular and muscle disorders. Importantly, some of the most commonly used prescription and over-the-counter medications, including aluminum antacids, beta-blockers, calcium channel blockers, anticholinergics, antidepressants, and opiates, cause defecatory dysfunction (Table 28.2). Lifestyle issues, such as inadequate fiber intake and insufficient fluid intake, can exert similar effects independently or in conjunction with other disorders.
Table 28.2 Drugs Associated with Constipation
| Over-the-Counter Medications | |
| Antidiarrheals (loperamide, Kaopectate) | |
| Antacids (with aluminum or calcium) | |
| Iron supplements | |
| Prescription Medications | |
| Anticholinergics | Others |
| Antidepressants | Iron |
| Antipsychotics | Barium sulfate |
| Antispasmodics | Metallic intoxication (arsenic, lead, mercury) |
| Antiparkinsonian drugs | Opiates |
| Antihypertensives | Nonsteroidal anti-inflammatory agents |
| Calcium channel blockers | Anticonvulsants |
| Beta-blockers | Vinca alkaloids |
| Diuretics | 5HT3 antagonists (ondansetron, granisetron) |
| Ganglionic blockers | |
Structural abnormalities refer to the obstructive disorders, such as pelvic organ prolapse, perineal descent, intussusception, rectal prolapse, and tumors. Functional disorders are those that do not have an identifiable anatomic or systemic etiology. Most functional disorders are motility disorders, such as slow-transit constipation or colonic inertia, irritable bowel syndrome (constipation predominant), and functional constipation. The Rome III criteria created strict definitions for these idiopathic conditions that are believed to result from the complex interaction of psychosocial factors and altered gut physiology via the gut-brain-gut axis (27). Patients also may have functional limitations, such as decreased mobility and cognition. It is important to understand that this classification system is somewhat arbitrary, and several of these conditions are interrelated.
Fecal Incontinence
Anal continence depends on a complex interaction of cognitive, anatomic, neurologic, and physiologic mechanisms. The continence mechanism can often compensate for a deficiency in one of these processes, but it can be overwhelmed with increased severity or decreased function over time. Systemic etiologies of fecal incontinence often are due to disease states that cause diarrhea. The rapid transport of large volumes of liquid stool to the rectum can produce urgency and incontinence even in healthy individuals (28). Fecal incontinence frequently results from infectious diarrhea caused by bacteria (e.g., Clostridium, Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), viruses (e.g., Rotavirus, Norwalk, HIV), and parasites (e.g., Entamoeba, Giardia, Cryptosporidium, Ascaris). Numerous medications and dietary items cause diarrhea and fecal incontinence (Table 28.3). Endocrine factors that can lead to fecal incontinence include diabetes mellitus and hyperthyroidism. With diabetes, diarrhea can develop from autonomic dysfunction, bacterial overgrowth, osmotic diarrhea with sugar substitutes, and pancreatic insufficiency. Inflammatory bowel disease is considered an idiopathic or autoimmune systemic factor. Ulcerative colitis and Crohn disease cause fecal incontinence during exacerbations with bouts of bloody diarrhea. Inflammatory bowel disease can also result in structural abnormalities, such as anal fissures, fistulas, abscesses, and operative complications that lead to fecal incontinence.
Table 28.3 Drugs and Dietary Items Associated with Diarrhea
| Over-the-Counter Medications | |
| Laxatives | |
| Antacids (with magnesium) | |
| Prescription Medications | |
| Laxatives | Chemotherapy |
| Diuretics | Colchicine |
| Thyroid preparations | Cholestyramine |
| Cholinergics | Neomycin |
| Prostaglandins | Para-aminosalicylic acid |
| Dietary Items | |
| Dietetic foods, candy or chewing gum, and elixirs with sorbitol, mannitol, or xylitol | |
| Olestra | |
| Caffeine | |
| Ethanol | |
| Monosodium glutamate | |
As with defecatory dysfunction, neurologic causes of fecal incontinence can be divided into central and peripheral disorders. Among the central nervous system disorders, upper motor neuron lesions above the level of the defecation center (located in the sacral cord) cause spastic bowel dysfunction. Cortical communication is disrupted, resulting in impaired cognitive control and sensory deficit. The anal sphincter is under spastic contraction, but digital stimulation can be performed to initiate reflex evacuation. Head trauma, neoplasms, and cerebral vascular accidents that damage portions of the frontal lobe result in loss of control of both micturition and defecation. Greater loss of inhibition is present when the lesion is located more anteriorly in the frontal lobe. Spinal cord trauma and lower motor neuron lesions above the defecation center tend to cause permanent loss of cortical control. For 2 to 4 weeks following spinal cord injury, “spinal shock” occurs, resulting in a temporary loss of reflexes below the level of the lesion, flaccid bowel function, constipation, and fecal impaction. After the initial shock, spastic paralysis ensues with hyperactive bowel function. The gastrocolic reflex, along with digital stimulation, initiates reflex evacuation in the absence of cortical inhibition. Fortunately, IAS tone is maintained despite the loss of EAS control for stress and urge situations. Both constipation and fecal incontinence can occur in these patients.
The demyelination that is seen in multiple sclerosis is randomly distributed and can occur at any level in the central nervous system. In addition to the somatic disruption that is similar to spinal cord injury, autonomic dysfunction frequently is present. People with dementia and other degenerative disorders that cause cognitive impairment frequently have fecal incontinence caused by overflow incontinence. Although sensory nerves are functioning properly, these individuals lack the cognitive awareness necessary to inhibit defecation until a socially acceptable time, and they develop overflow incontinence.
Lower motor neuron lesions occurring at or below the level of the defecation center in the sacral cord cause flaccid bowel dysfunction. Cortical communication is disrupted, resulting in impaired cognitive control and sensory deficit. The bowel reflexes, including the bulbocavernosus and anal reflexes, are interrupted. The anal sphincter is flaccid, and fecal retention with overflow incontinence usually occurs. Digital disimpaction and Valsalva often are required for evacuation. Digital stimulation has no effect, and medications tend to work poorly. Examples of motor neuron lesions include tumor or trauma to the cauda equina, tabes dorsalis, spina bifida, and peripheral neuropathy.
The classic example of peripheral neuropathy is congenital aganglionosis (Hirschsprung disease), which was discussed earlier. The most common peripheral neuropathy occurs with diabetes. Approximately 20% of individuals with diabetes have fecal incontinence (29). The cause tends to be multifactorial with the exact mechanism uncertain. Fecal incontinence can occur with diabetic diarrhea or years later from progressive disease. Individuals with diabetes frequently experience intestinal autonomic neuropathy, an abnormal gastrocolic reflex, and chronic constipation. The subsequent pelvic floor denervation causes fecal incontinence by sensory neuropathy, failure of the rectoanal inhibitory reflex, and sphincter dysfunction (30). Consequently, fecal incontinence from peripheral neuropathy can be the result of defective sampling, a disrupted rectoanal inhibitory reflex, or pudendal neuropathy with sphincter dysfunction. Patients may experience stress or urge incontinence as well as overflow incontinence.
Anatomic and structural causes of fecal incontinence are usually due to obstetric or surgical trauma. Damage or dysfunction of the IAS, EAS, and puborectalis can result in varying degrees of fecal incontinence. Those with impaired resting tone from a defective IAS will have passive incontinence (incontinence at rest), which is worse during sleep because of decreased EAS activity (31). An inability to respond to sudden distention and to suppress defecation is often seen with external sphincter dysfunction. External and internal sphincter dysfunction often causes incontinence of liquid stool. Incontinence of solid stool is usually seen with widening of the anorectal angle from damage to the puborectalis muscles. Damage to the anal cushions usually causes minor soiling. Other anatomic and structural abnormalities associated with fecal incontinence include obstructive disorders such as pelvic organ prolapse, descending perineum syndrome, anismus, and intussusception; fistulas from diverticulitis, inflammatory bowel disease, cancer, or surgical trauma; and decreased rectal compliance from inflammatory bowel disease, cancer, and radiation. Decreased compliance results in higher intraluminal pressures with smaller volumes of stool, poor storage capacity, urgency, and incontinence (32).
Functional disorders associated with fecal incontinence include irritable bowel syndrome (diarrhea variant), functional diarrhea, decreased mobility, and decreased cognition.
Combined Disorders of Defecation and Fecal Incontinence
Several conditions have the potential to cause both defecatory dysfunction and fecal incontinence (Table 28.1). Most of these disorders cause combined symptoms through the development of fecal impaction followed by overflow incontinence. This situation can be seen with many of the neurologic conditions, pelvic outlet obstructive disorders, functional disorders of irritable bowel syndrome, decreased mobility, and decreased cognition. The cause of these symptoms is often multifactorial.
Structural versus Functional Disorders
Disordered Defecation
Disordered defecation can result from outlet obstruction or functional motility disorders.
Outlet Obstruction
Anismus/Rectosphincteric Dyssynergia
Anismus is otherwise known as rectosphincteric dyssynergia, pelvic floor dyssynergia, spastic floor syndrome, and paradoxical puborectalis syndrome. The anorectal angle narrows as a result of paradoxical contraction of the puborectalis and external anal sphincter during defecation. Frequent symptoms include dyschezia, straining, hard stools, incomplete emptying, and tenesmus. A recent prospective study of 120 patients with dyssynergic defecation found a higher prevalence in women (77%) (33,34). The need for digital assistance (digital disimpaction or splinting) to evacuate the rectum occurs in up to 58% of patients. Psychosocial factors, such as a history of sexual abuse, depression, eating disorder, obsessive-compulsive disorder, and stress, may play an important role in this disease. In this study, 22% reported a history of sexual abuse, and 31% reported a history of physical abuse. One-third believed the problem began during childhood, and 24% reported a precipitating illness or surgery was related to a particular event. Five percent of women claimed that pregnancy or childbirth was a precipitating factor. This condition also is seen in young children with constipation and dyschezia. The response to biofeedback and pelvic floor physical therapy, as well as the aforementioned patient characteristics, indicate a learned response mechanism is involved (33,34). Although this is often categorized as an outlet obstruction, the Rome III criteria for functional gastrointestinal disorders places this in the category of functional defecation disorders. The specific Rome III diagnostic criteria for dyssynergic defecation includes “inappropriate contraction of the pelvic floor or less than 20% relaxation of basal resting sphincter pressure with adequate propulsive forces during attempted defecation” (35).
Pelvic Organ Prolapse
Pelvic organ prolapse bears special mention because it is often seen by gynecologists but inconsistently associated with defecatory dysfunction. Prolapse is very common, although many women with this condition are asymptomatic. Those with symptoms may report incomplete evacuation and the need to apply digital pressure to the posterior vaginal wall or perineum to aid in evacuation of stool (digitation or splinting). It is important to rule out other causes of constipation, because these symptoms are nonspecific, and rectocele can result from chronic straining and increased intra-abdominal pressure due to other etiologies of defecatory dysfunction. Defecatory dysfunction related to pelvic organ prolapse can result from rectocele, enterocele, or perineal descent, either individually or in combination.
Rectocele is a herniation of the rectal mucosa through a defect in the rectovaginal septum. These site-specific defects can be transverse or longitudinal through the inferior, middle, or superior regions of the rectovaginal septum (36). Enterocele is a herniation of a peritoneal sac and bowel through the pelvic floor, typically between the uterus or vaginal cuff and rectum. It is more common following hysterectomy and retropubic urethropexy. There are two theories surrounding the formation of an enterocele. The first theory implicates a defect in the fibromuscular endopelvic fascia of the vagina, allowing peritoneum and bowel to herniate. The second theory attributes its formation to a support defect with full thickness protrusion, including endopelvic fascia (37). Ultimately, the mechanism might be attributed to a combination of the two theories because some support defects are secondary to superior breaks in the rectovaginal and pubocervical fascia. Patients with rectocele and enterocele may have similar symptoms, including pelvic pressure, vaginal protrusion, obstipation, fecal incontinence, and sexual dysfunction. Although associations have been made between defecatory dysfunction and advanced stages of pelvic organ prolapse, a causal relationship remains to be established. Controversy remains as to whether anatomic herniation is the cause of these symptoms or the effect of underlying colonic dysfunction, chronic constipation, and straining.
Descending perineum syndrome is defined as descent of the perineum (at the level of the anal verge) beyond the ischial tuberosities during Valsalva. Excessive perineal descent was first described in the colorectal literature by Parks et al. in 1966 (38,39). It occurs as a result of inferior detachment of the rectovaginal septum from the perineal body. As the condition progresses, the patient can develop pudendal neuropathy from stretch injury. Perineal descent has been associated with a variety of defecatory disorders, including constipation, fecal incontinence, rectal pain, solitary rectal ulcer syndrome, rectocele, and enterocele (40).
Rectal Intussusception
Rectal intussusception or intrarectal prolapse is the circumferential prolapse of the upper rectal wall into the rectal ampulla but not through the anal verge. It occurs most often in women in their fourth and fifth decades. The most common symptoms are obstructive, including incomplete emptying, manual disimpaction, splinting, pain with defecation, and bleeding. Other symptoms include fecal incontinence, decreased urge to defecate, inability to distinguish between gas and feces, and mucus discharge with pruritus ani. Bleeding often originates from a solitary rectal ulcer or localized proctitis of the involved bowel segment (41). Intussusception is seen in as many as one-third of women with defecatory dysfunction and other symptoms, such as constipation, rectal pain, and fecal incontinence (42). It has also been seen in 29% of asymptomatic patients (43). The intussusception rarely develops into total rectal prolapse (44).
Functional Motility Disorders
Functional Bowel Disorders
Functional bowel disorders, as defined by the Rome III criteria, consist of irritable bowel syndrome, functional bloating, functional constipation, functional diarrhea, and unspecified functional bowel disorders. In this section we will focus primarily on irritable bowel syndrome (45).
Irritable bowel syndrome (IBS) has been estimated to have a prevalence of 10% to 20% and is more common in women and younger individuals. It accounts for 25% to 50% of all referrals to gastrointestinal clinics. Irritable bowel syndrome has distinct diagnostic criteria, including the exclusion of structural or metabolic abnormalities. These patients often have other gastrointestinal, genitourinary, and psychological illness, including gastroesophageal reflux disease, fibromyalgia, headache, backache, chronic pelvic pain, sexual dysfunction, lower urinary tract dysfunction, depression, and anxiety. Stressful life events seem to correlate with the onset and exacerbation of symptoms. A detailed history frequently reveals past physical or sexual abuse (46). Currently, specific criteria allow for classification of IBS into diarrhea-, constipation-, and pain-predominant categories (Table 28.4). The constipation variant is most commonly associated with defecatory dysfunction, whereas the diarrhea variant causes fecal incontinence. The pain or spastic variant causes predominantly abdominal discomfort but can also be associated with both defecatory dysfunction and fecal incontinence. After excluding organic disease, the criteria listed in Table 28.4 have a sensitivity of 65%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 76% (47).
Table 28.4 Irritable Bowel Syndrome
| Diagnostic Criteriona |
| Recurrent abdominal pain or discomfortb at least 3 days per month in the last 3 months associated with two or more of the following: |
1. Improved with defecation 2. Onset associated with a change in frequency of stool 3. Onset associated with a change in form (appearance) of stool |
aCriterion fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis. b“Discomfort” means an uncomfortable sensation not described as pain. |
| In pathophysiology research and clinical trials, a pain/discomfort frequency of at least 2 days a week during the screening evaluation is recommended for subject eligibility. |
| From Drossman DA, Corazziari E, Talley NJ, et al., eds. Rome III: the functional gastrointestinal disorders. 3nd ed. McLean, VA: Degnon Associates, 2006:885–897, Appendix A, with permission. |
Functional constipation is a term created by the Rome II criteria as a unifying definition of constipation (Table 28.5). The rationale for the criteria listed in Table 28.5 stems from the variability in patient definitions of constipation (46).
Table 28.5 Functional Constipation
| Diagnostic Criteriaa* |
1. Must include two or more of the following: a. Straining during at least 25% of defecations b. Lumpy or hard stools in at least 25% of defecations c. Sensation of incomplete evacuation for at least 25% of defecations d. Sensation of anorectal obstruction/blockage for at least 25% of defecations e. Manual maneuvers to facilitate at least 25% of defecations (e.g., digital evacuation, support of the pelvic floor) f. Fewer than three defecations per week 2. Loose stools are rarely present without the use of laxatives, and there are insufficient criteria for IBS. 3. Insufficient criteria for irritable bowel syndrome |
aCriteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis |
| From Drossman DA, Corazziari E, Talley NJ, et al., eds. Rome III: the functional gastrointestinal disorders. 3nd ed. McLean, VA: Degnon Associates, 2006:885–897, Appendix A, with permission. |
Functional Defecation Disorders
Functional defecation disorders are divided into dyssynergic defecation and inadequate defecatory propulsion (colonic inertia). (For the purposes of this chapter, dyssynergic defecation has been included in the structural category of outlet obstruction; however, it is important to recognize that Rome III considers it a functional disorder.) Both of the functional defecation disorders require the presence of functional constipation. Table 28.6 lists the criteria for diagnosing these conditions.
Table 28.6 Functional Defecation Disorders
| Diagnostic Criteriaa |
1. The patient must satisfy diagnostic criteria for functional constipation (Table 28.5) 2. During repeated attempts to defecate must have at least two of the following: a. Evidence of impaired evacuation, based on balloon expulsion test or imaging b. Inappropriate contraction of the pelvic floor muscles (i.e., anal sphincter or puborectalis) or less than 20% relaxation of basal resting sphincter pressure by manometry, imaging, or EMG c. Inadequate propulsive forces assessed by manometry or imaging |
aCriteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis |
| From Drossman DA, Corazziari E, Talley NJ, et al., eds. Rome III: the functional gastrointestinal disorders. 3nd ed. McLean, VA: Degnon Associates, 2006:885–897, Appendix A, with permission. |
Colonic Inertia/Slow-Transit Constipation
Severe constipation, defined as fewer than three stools per week and refractory to therapy, is relatively rare; however, these patients frequently suffer from motility disorders such as global motility disorder and colonic inertia. Women are more likely to be affected than men. Colonic inertia or slow-transit constipation is defined as the delayed passage of radiopaque markers through the proximal colon without retropulsion of markers from the left colon and in the absence of systemic or obstructive disorders. The cause remains unclear. Patients with this disorder have impaired phasic colonic motor activity and diminished gastrocolic reflexes (48,49). Studies on the role of laxatives, absorption, hormones, psychological abnormalities, and endogenous opioids have been inconclusive. Current literature suggests a possible neurologic or smooth muscle disorder (49,50).
Fecal Incontinence
Sphincter Disruption
In young women, obstetric injury is the most common cause of fecal incontinence. The mechanism of injury can be from anatomic disruption of the anal sphincter complex, pelvic floor denervation, or a combination of the two conditions. The risk factors for anal sphincter laceration are primiparity, high birth weight, forceps delivery, and episiotomy (51–53). Recent work suggests that women with anal sphincter injuries have slower labor, without the normal deceleration phase, and with late descent of the fetal head (54). Although there are limited long-term prospective studies demonstrating the natural history of anal sphincter injury, pelvic floor neuropathy, and the progression of these conditions to fecal incontinence, current literature supports the relationship of early-onset symptoms to sphincter damage and delayed-onset symptoms to neuropathy (55). This relationship would account for the large discrepancy in the prevalence of fecal incontinence between younger men and women that decreases as the population ages (56).
Obstetric Trauma
Third- and fourth-degree lacerations at delivery are associated with an increased risk of fecal incontinence (odds ratio [OR] 3.09)[MB2] (55). Whereas the incidence of clinically documented third- and fourth-degree anal sphincter tears is between 0.5% and 5.9% (51,53,57), occult third- and fourth-degree defects are present in 28% to 35% of primiparous women and 44% of multiparous women, and approximately one-third of these patients have symptoms of anal incontinence. Patients with occult anal sphincter tears are 8.8 times more likely to have fecal incontinence (53,58). Forceps-assisted vaginal delivery significantly increases this risk, but the data on vacuum-assisted delivery are less conclusive (52,59,60). Elective cesarean delivery, in contrast with emergency cesarean delivery, was believed to prevent anal incontinence, but recent studies argue against any protective effect with cesarean delivery, irrespective of timing (46,51,53,59,61,62). A recent Cochrane review concludes that there is insufficient evidence to support primary elective cesarean delivery for the purpose of preserving fecal continence (63). Midline episiotomy is strongly linked to sphincter damage and fecal incontinence (52,64). One study of a large population found conflicting results, with an overall protective effect seen with episiotomy (OR 0.89). The likelihood of fourth-degree laceration was increased (OR 1.12) and of third-degree laceration was decreased (OR 0.81) (51). A Cochrane review supports the restrictive use of both midline and mediolateral episiotomy due to less posterior perineal trauma, less suturing, and fewer healing complications. There were no differences in severe trauma, pain, dyspareunia, or urinary incontinence, but there was an increase in anterior perineal trauma with restrictive use (65). An important finding in another study was that one-half of patients who underwent immediate repair of a third-degree laceration had symptoms of anal incontinence, and 85% had persistent sphincter defects on endoanal ultrasonography (66).
Surgical Trauma
Iatrogenic injury follows obstetric trauma as the second most common cause of direct sphincter damage. Surgical procedures that have been associated with fecal incontinence include anal fistula repair, anal sphincterotomy, hemorrhoidectomy, and anal dilation. Fistulotomy is the most common procedure that results in fecal incontinence. Rectovaginal or anovaginal fistulas can develop after obstetric injury, operative complications during pelvic surgery, and inflammatory bowel disease exacerbations. Fistulas cause fecal incontinence, and the degree of postoperative dysfunction depends on the location of the fistula and the amount of sphincter that is disrupted during the surgical repair. It also depends on the preoperative level of sphincter function and pudendal nerve function. Anal sphincterotomy to treat painful anal fissures can lead to incontinence by disruption of rectal sensory innervation and anal cushions and transection of the anal sphincter (67,68). Hemorrhoidectomy often results in minor soiling as a result of resection of the anal cushions, which act as the final mucosal barrier. Similar to sphincterotomy, rectal sensory innervation can be disrupted, and injury to the internal sphincter can occur during sharp dissection (68,69).
Sphincter Denervation
Idiopathic (primary neurogenic) fecal incontinence results from denervation of both the anal sphincter and pelvic floor muscles. Denervation injury related to obstetric trauma accounts for approximately three of four cases of idiopathic fecal incontinence and is the most common overall cause of fecal incontinence (70,71).
Obstetric Trauma
The two proposed mechanisms of pudendal neuropathy are stretch injury during the second stage of labor and compression of the nerve as it exits Alcock’s canal (70). Established risk factors for pelvic floor neuropathy include multiparity, high birth weight, forceps delivery, prolonged active second stage, and third-degree laceration (72,73). Several studies have shown increased pudendal nerve terminal motor latencies following vaginal delivery, especially after sphincter laceration (53,71,74). Most women will recover function within a few months postpartum. Others will have evidence of injury several years later, which may represent the cumulative effects of subsequent deliveries (71,75). However, fecal incontinence will develop in only a fraction of patients with neuropathy (73).
Descending Perineum Syndrome
As noted previously, prolonged straining for any reason could cause descending perineum syndrome. This syndrome is defined as descent of the perineum beyond the ischial tuberosities during Valsalva (38,39). Pudendal neuropathy results from stretching and entrapment of the pudendal nerve. This diagnosis is supported by findings of elongation of the pudendal nerve, prolonged pudendal nerve motor terminal latency, and decreased anal sensation in women with perineal descent (76–78). As pudendal neuropathy progresses, it ultimately leads to fecal incontinence (40,79).
Functional Bowel Disorders
Functional Fecal Incontinence
The Rome III criteria established well-defined guidelines for functional causes of fecal incontinence (Table 28.7). The criteria essentially exclude systemic and anatomic abnormalities; however, minor abnormalities of sphincter innervation or structure are permitted.
Table 28.7 Functional Fecal Incontinence
| Diagnostic Criteriaa |
1. Recurrent uncontrolled passage of fecal material in an individual with a developmental age of at least 4 years and one or more of the following: a. Abnormal functioning of normally innervated and structurally intact muscles b. Minor abnormalities of sphincter structure and/or innervation c. Normal or disordered bowel habits, (i.e., fecal retention or diarrhea) d. Psychological causes AND 2. Exclusion of all of the following a. Abnormal innervation caused by lesion(s) within the brain (e.g., dementia), spinal cord, or sacral nerve roots, or mixed lesions (e.g., multiple sclerosis), or as part of a generalized peripheral or autonomic neuropathy (e.g., due to diabetes) b. Anal sphincter abnormalities associated with a multisystem disease (e.g., scleroderma) c. Structural or neurogenic abnormalities believed to be the major or primary cause of fecal incontinence. |
aCriteria fulfilled for the last 3 months |
| From Drossman DA, Corazziari E, Talley NJ, et al., eds. Rome III: the functional gastrointestinal disorders. 3nd ed. McLean, VA: Degnon Associates, 2006:885–897, Appendix A, with permission. |
Irritable Bowel Syndrome
The diarrhea variant of irritable bowel syndrome is often associated with fecal incontinence as well as disordered defecation. The criteria for diagnosis are presented in Table 28.4.
Functional Diarrhea
The Rome III criteria create a unifying definition of diarrhea called functional diarrhea (Table 28.8). The rationale for the criteria listed in Table 28.8 stems from the variability in patients’ descriptions of diarrhea (46).
Table 28.8 Functional Diarrhea
| Diagnostic Criteriona |
| Loose (mushy) or watery stools without pain occurring in at least 75% of stools |
aCriteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis |
| From Drossman DA, Corazziari E, Talley NJ, et al., eds. Rome III: the functional gastrointestinal disorders. 3nd ed. McLean, VA: Degnon Associates, 2006:885–897, Appendix A, with permission. |
Pitfalls for the Pelvic Floor Surgeon
It sometimes is easy to overlook or misinterpret signs and symptoms of constipation and defecatory dysfunction. Any acute change in bowel habits must be evaluated thoroughly, and malignancy must be considered in the differential diagnosis. Even in the presence of chronic disease, malignancy must still be excluded. Persistent symptoms after an empiric trial of medical therapy should prompt further evaluation, including colonoscopy or flexible sigmoidoscopy. It is also possible to mistakenly attribute symptoms of defecatory dysfunction and constipation to pelvic organ prolapse when prolapse is actually the result of an underlying bowel disorder. In this case, surgical treatment of prolapse will have little lasting benefit if the underlying bowel disorder remains untreated.
History and Physical Examination
History
A thorough history and physical examination are critical to the evaluation of fecal incontinence and defecatory dysfunction. The history of present illness should focus on the bowel habits, including frequency and consistency of bowel movements (hard vs. soft, formed vs. unformed, diarrhea vs. constipation). Determining the duration and severity of symptoms, as well as exacerbating factors, is important for understanding the impact on quality of life. Patients should be questioned about straining with bowel movements, symptoms of incomplete emptying, and splinting of the perianal region, perineal body, or posterior vaginal wall to assist with evacuation. Patients should also be asked about the need to perform digital disimpaction because they are unlikely to volunteer this information. With respect to fecal incontinence, information should be obtained about leakage with solids, liquid, and flatus and the ability to discriminate between these different types of stool (sampling). Similar to urinary incontinence, fecal incontinence can be stress related, urge related, or unconscious. Questions about alternating diarrhea and constipation, mucus or blood in the stools, constitutional symptoms, and changes in stool caliber can help the investigator uncover systemic and functional etiologies. Finally, it is important to ask about adaptive behaviors, incontinence product usage, and past and present treatments, including surgery, physical therapy, and medications.
A large amount of information can be obtained efficiently through questionnaires. Validated questionnaires quantify symptoms, which are subjective in nature, to objectively measure response to treatment. A valuable survey to assess defecatory dysfunction is the Colorectal-Anal Distress Inventory (CRADI), which has been incorporated into the Pelvic Floor Distress Inventory (PFDI) (80). The latter is a useful tool for evaluating symptoms of prolapse, urinary incontinence, fecal incontinence, voiding dysfunction, and defecatory dysfunction. Other useful symptom scales and bother scores for fecal incontinence include the Wexner Score, Fecal Incontinence Severity Index, and Fecal Incontinence Quality of Life Scale (81–83).
The medical history, surgical history, family history, and review of systems should focus on uncovering potential systemic and obstructive disorders shown in Table 28.1. A complete obstetric history should include the number of vaginal deliveries, operative vaginal deliveries, or presence of a third- or fourth-degree laceration, which is critical for patients with fecal incontinence. Length of the second stage of labor, birth weight, and the use of episiotomy should be ascertained because they may pose risk factors for sphincter damage and denervation. The sexual history should include questions about rape, anal intercourse, and dyspareunia. Use of over-the-counter, prescription, and illegal drugs should be recorded as well as food allergies.
Physical Examination
The evaluation of anorectal dysfunction requires a basic general examination as well as a focused abdominal and pelvic examination. The general physical survey should include a global assessment of mobility and cognitive function. Routine examination of the abdomen involves inspection, palpation, and auscultation to rule out the presence of masses, organomegaly, and areas of peritoneal irritation. This examination should be followed by a detailed evaluation of the vagina, perineum, and anorectum. The goals of the pelvic examination are to define objectively the degree of prolapse and determine the integrity of the connective tissue, neurologic function, and muscular support of the pelvic organs.
Neurologic Examination
Important elements of the neurologic examination are assessment of cranial nerve function, sensation and strength of the lower extremities, and reflexes for the lower extremities, bulbocavernosus, and anal wink. These examinations evaluate the function of the lower lumbar and sacral nerve roots, recognizing the importance of the second through fourth sacral nerve roots in pelvic floor dysfunction. The perineal reflexes can be elicited by stroking the labia majora and perianal skin or tapping the clitoris with a cotton-tipped swab. The anal wink, bulbocavernosus, and cough reflexes all test the integrity of motor innervation to the external anal sphincter (S2–4). Sensation over the inner thigh, vulva, and perirectal areas should be tested for symmetry to light touch and pinprick.
Muscle Strength
The integrity of the pelvic floor muscles should be assessed at rest and with voluntary contraction to determine strength, duration, and anterior lift. The ability to relax these muscles and tenderness on palpation should also be evaluated. Several standardized systems have been described to objectively measure muscle strength, but none has been accepted as a standard. The puborectalis muscle should be readily palpable posteriorly as it creates a 90-degree angle between the anal and rectal canals. Voluntary contraction of this muscle “lifts” the examining finger anteriorly toward the pubic rami. An intact external anal sphincter muscle that has decreased tone and contractility often indicates pudendal neuropathy. Similarly, neuropathy affecting the puborectalis can be recognized by an obtuse anorectal angle and weak voluntary contraction. Similar to the urethral axis, the anorectal angle can also be tested using a cotton-tipped swab, although this test is rarely performed. Deflection is measured in the supine position at rest, with strain, and with squeeze.
Vaginal Support
The salient points of pelvic organ prolapse (see Chapter 27 for patients with defecatory dysfunction are the support of the vaginal apex, posterior wall, and perineal body, although some experts believe anterior wall defects can also affect defecatory dysfunction. The posterior wall is assessed while supporting the vaginal apex and anterior wall with a Sims speculum. This permits the examiner to focus on identifying specific locations of rectovaginal fascial defects. A rectovaginal examination aids in identification of defects in the rectovaginal fascia or perineal body. Loss of vaginal rugation has also been reported overlying the site of a rectovaginal fascial tear (84). This technique is especially useful for enteroceles, which have a smooth, thin epithelium over the enterocele sac or peritoneum.
Normally, the perineum should be located at the level of the ischial tuberosities, or within 2 cm of this landmark. A perineum below this level, either at rest or with straining, represents perineal descent. Subjective findings of perineal descent include widening of the genital hiatus and perineal body, as well as a flattening of or a convex appearance of the intergluteal sulcus. Women with perineal descent also tend to have less severe stages of pelvic organ prolapse based on the Pelvic Organ Prolapse Quantification (POP-Q) staging system because it measures descent from the hymenal ring (85). An increase in the length of the perineal body and genital hiatus consistent with straining suggests perineal descent. The degree of perineal descent can also be measured objectively with a St. Mark’s perineometer, although a thin ruler placed in the posterior introitus at the level of the ischial tuberosities also can be used. Descent is measured as the distance the perineal body moves when the patient strains. Although pelvic floor fluoroscopy is the standard technique for measuring perineal descent, this technique is most useful in patients with symptoms of severe defecatory dysfunction and evidence of perineal descent on pelvic examination.
Anorectal Examination
Visual and digital inspection of the vagina and anus will help to identify structural abnormalities such as prolapse, fistulas, fissures, hemorrhoids, or prior trauma. As previously mentioned, a rectovaginal examination provides useful information regarding the integrity of the rectovaginal septum and can demonstrate laxity in the support of the perineal body. The rectovaginal examination is helpful in the diagnosis of enteroceles, which can be felt as protrusion of bowel between the vaginal and rectal fingers with straining. Digital rectal examination should be performed at rest, with squeeze, and while straining. The presence of fecal material in the anal canal may suggest fecal impaction or neuromuscular weakness of the anal continence mechanism. Circumferential protrusion of the upper rectum around the examining finger during straining suggests intussusception, which often occurs in combination with laxity of the posterior rectal support along the sacrum.
The integrity of the external anal sphincter and puborectalis muscle can be evaluated by observation and palpation of these structures during voluntary contraction. Evidence of dovetailing of the perianal skin folds and the presence of a perineal scar with an asymmetric contraction often indicates a sphincter defect. When a patient is asked to contract her pelvic floor muscles, two motions should be present: The external anal sphincter should contract concentrically, and the anal verge should be pulled inward. These actions should also be apparent on digital rectal examination. As mentioned previously, the 90-degree angle created by the puborectalis should be readily palpable posteriorly and, with voluntary contraction, the examining finger should be lifted anteriorly toward the pubic rami. Both the puborectalis and external anal sphincter should relax during Valsalva effort. Patients with anismus may experience a paradoxical contraction of these muscles during straining. Finally, defects in the anterior aspects of the external anal sphincter may be detected by digital examination.
Testing
Sophisticated diagnostic testing is currently being used in clinical research and in anorectal physiology laboratories to quantify the function of the colon and anorectum. Following is a description of these techniques as they relate to the management of fecal incontinence and disordered defecation.
Fecal Incontinence
Endoanal Ultrasonography
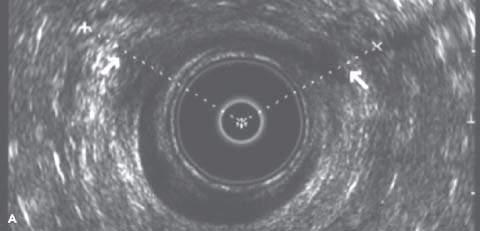
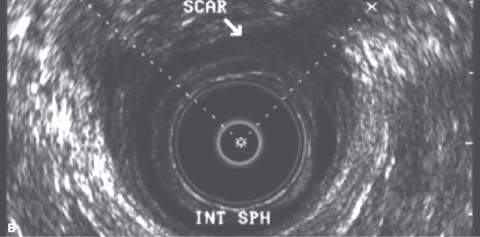
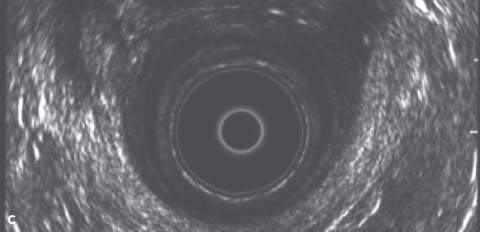
Endoanal ultrasonography permits accurate imaging of both the internal and external anal sphincters. It can assess the continuity and thickness of the muscle and currently is considered the single best method for detecting anal sphincter defects. Endoanal ultrasonography is performed using a Bruel-Kjaer (Copenhagen, Denmark) ultrasound scanner with a 360-degree rectal endoprobe (type 1850) with a 7.0 MHz transducer (focal length, 2–5 cm) housed within a plastic cone (Fig. 28.1). The normal IAS is a continuous hypoechoic band of smooth muscle surrounded by the thick echogenic layer of the striated EAS. A sphincter defect occurs when there is disruption in these muscle bands. Location and severity of the defect can be described by circumferential distance in degrees, percentage of thickness, and distance from the anal verge (Fig. 28.2). Measurements are usually taken in the proximal, middle, and distal anal canal. It is important to recognize the physiologic split in the proximal EAS as it merges with the puborectalis muscle of the levator ani. Misinterpretation of this finding as a sphincter defect can result in an increased prevalence of reported defects. The puborectalis muscle appears as a U-shaped or V-shaped thick echogenic layer outside the IAS in the proximal anal canal. Magnetic resonance imaging (MRI) may be equally as effective or better at diagnosing sphincter defects, especially with the use of a vaginal or rectal coil. For this purpose, MRI is more expensive, and currently its use is largely investigational. It may be beneficial in cases in which endoanal ultrasonography results are inconclusive or the quality of the study is poor.
Figure 28.1 Bruel-Kjaer (Copenhagen, Denmark) ultrasound probe (type 1850) with a 7.0 MHz transducer (focal length, 2 to 5 cm) housed with a plastic cone.

Figure 28.2 A: Endoanal ultrasound image from the distal anal canal demonstrating defects in the internal sphincter from 10 to 3 o’clock and the external sphincter from 10 to 2 o’clock. B: Endoanal ultrasound image from the middle anal canal demonstrating defects in the internal sphincter from 12 to 2 o’clock and the external sphincter from 10 to 1 o’clock. C: Endoanal ultrasound image from the proximal anal canal demonstrating an intact IAS and a normal physiologic split in the external sphincter.
Electromyography
Electromyography (EMG) is used to evaluate neuromuscular integrity of the EAS following a traumatic injury such as childbirth, as well as to document the presence of pelvic floor neuropathy (86). This technique measures the electrical activity arising in muscle fibers during contraction and at rest. Different types of electrodes may be employed, including surface electrodes, concentric needle electrodes, and single-fiber electrodes. Surface electrodes are less invasive because they are applied near or within the anal canal, but they are capable only of recording basic anal sphincter activity. This technique often is used in conjunction with biofeedback therapy. Concentric needle electrodes are most commonly used in anorectal physiology laboratories to selectively survey an individual muscle’s activity. Insertion of the thin needlelike cannulas containing steel wire electrodes can be painful. Even smaller single-fiber EMG electrodes are used to record the activity of single muscle fibers, which can be quantified to calculate fiber density. Following denervation injury, increased muscle fiber density occurs during reinnervation. Thus, single-fiber EMG can provide indirect evidence of neurologic injury by mapping the EAS and identifying injured areas. This technique is used rarely in clinical practice. Endoanal ultrasonography offers increased patient comfort and more reliable results than EMG and has replaced this technique for the detection of EAS disruption because of increased patient comfort and more reliable results.
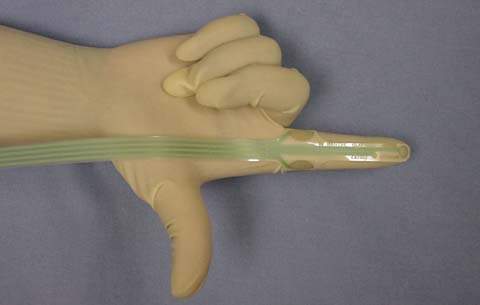
Motor nerve conduction studies provide another means of measuring pelvic floor neuropathy. The axon of a nerve is stimulated, and the time it takes the action potential to reach the muscle supplied by the nerve is recorded. The delay between stimulation and the muscle response is called the nerve latency. Pudendal nerve terminal motor latency (PNTML) can be determined by transrectal stimulation of the pudendal nerve using a St. Mark’s electrode (87). A nerve stimulator is mounted on an examination glove at the fingertip (Fig. 28.3) and positioned transrectally over each ischial spine. A stimulus of up to 50 mV for 0.1 milliseconds is applied, and the latency of the EAS muscle contraction is measured. A value of 2.2 milliseconds or less is considered normal. A recent study evaluating normative values for pudendal and perineal nerve latencies observed increased latencies with increased age (88). Prolongation of the PNTML is indicative of damage to that nerve or the presence of a demyelinating condition. Pudendal nerve function has prognostic value in the surgical repair of traumatic sphincter injuries and is useful in preoperative counseling (89).
Figure 28.3 St. Mark’s electrode used for measuring pudendal nerve motor terminal latency. The stimulating electrode is on the fingertip, and the receiving electrode is on the proximal finger near the knuckle.
Anal Manometry
Anal manometry is used to quantify function of the anal sphincter mechanism. Water-perfused manometry catheters or water-filled balloons are most often used to measure anal canal pressures. Resting anal canal pressures reflect IAS function, and pressures in the lower anal canal during maximal voluntary contraction reflect EAS function. Vector analysis can be used to detect asymmetry within the anal sphincter. Anal manometry provides indirect evidence of sphincter injury; low resting tone indicates IAS injury, and decreased maximum squeeze pressure indicates EAS injury. Anal pressures are influenced by a variety of factors, including tissue compliance and muscular tone. Consequently, anal manometry results are difficult to interpret and correlate poorly with the specific anatomic defect. Interpretation is further complicated by the wide variation of normal pressure values that change with age and parity. Significant overlaps occur between manometric values for incontinent patients and those without incontinence. Thus, anal manometry may be of limited value in the evaluation and treatment of anal sphincter defects and fecal incontinence.
Proctoscopy and Flat Tire Test
Proctoscopy has an important role in the evaluation of fecal incontinence. It can be performed independently or during colonoscopy, flexible sigmoidoscopy, and the flat tire test. Proctoscopy can detect anorectal pathology, such as prolapsing hemorrhoids, intussusception, ulcerative or radiation proctitis, or a solitary rectal ulcer. The flat tire test is added when a rectovaginal or colovaginal fistula is suspected but cannot be visualized on routine office evaluation. This test usually is performed under anesthesia but can also be done in the office setting. Saline or water is placed in the vagina with the patient in Trendelenburg position. Using a proctoscope or rigid sigmoidoscope, air is instilled into the rectum. Vaginal retractors provide visualization of the posterior vaginal epithelium and vaginal apex. Observation of bubbling into the vaginal fluid confirms the diagnosis and location of a rectovaginal or colovaginal fistula. The rectal site of the fistula usually is identifiable, depending on the size and location of the fistula as well as the quality of the bowel preparation.
Disordered Defecation
Sitzmark Study
Colonic transit studies are performed using ingested radiopaque markers followed by serial abdominal radiography. Patients are asked to follow a high-fiber diet over the test period and avoid the use of laxatives, suppositories, or enemas. A capsule containing 20 to 24 markers is ingested initially, and abdominal radiography is performed either daily or on the fourth day, the seventh day, and every 3 days thereafter until all the markers are gone. Segmental transit times are then calculated using a mathematical formula. Colonic transit study results are used to classify patients with constipation into delayed transit, normal transit, and outlet obstruction. After day 6, there should be fewer than five markers remaining in the colon. With slow transit, more than five markers are scattered throughout the colon. With outlet obstruction, more than five markers are in the rectosigmoid region, and transit is normal throughout the rest of the colon.
Pelvic Floor Fluoroscopy and Magnetic Resonance Imaging
Pelvic fluoroscopy permits radiological evaluation of pelvic floor and anorectal anatomy and physiology. It is particularly useful in obstructive defecation disorders, such as intussusception, rectocele, enterocele, anismus, and perineal descent. The patient is placed on a radiolucent commode, and contrast material is instilled into the rectum. The addition of vaginal, bladder, and oral contrast material is helpful diagnostically when multicompartmental prolapse is suspected. A series of lateral still images or continuous imaging using videography are made with fluoroscopy while the patient is at rest, during defecation, and with contraction of the anal sphincter. Similar films can be obtained for evacuation of the bladder. Pelvic fluoroscopy has many names, including defecography, defecating proctography, defecating cystoproctography, and colpocystoproctography, depending on the technique used. The measurements obtained include size of the rectal ampulla, length of the anal canal, anorectal angle, puborectalis motion, and pelvic floor descent. Severity of prolapse and pelvic floor descent is quantified in relation to the pubococcygeal line. Pelvic fluoroscopy is superior to physical examination for diagnosing enterocele, and this technique has the advantage of being able to distinguish enteroceles from sigmoidoceles (90). Rectosphincteric dyssynergia may be present when the patient experiences incomplete relaxation of the puborectalis muscle during rectal evacuation, the anorectal angle is preserved, and there is incomplete emptying. Pelvic fluoroscopy is considered the definitive test for diagnosing intussusception, and it is the preferred technique for quantifying perineal descent (91).
Dynamic MRI with luminal contrast is an imaging modality similar to pelvic fluoroscopy. Its ability to detect prolapse is similar to that of fluoroscopy, but MRI can visualize pelvic floor musculature and soft tissue, thus giving it the advantage of detecting ballooning of the levator muscles and levator ani hernias. The supine position of the testing is a drawback; however, there are isolated reports of upright dynamic MRI using open scanners that show results comparable to fluoroscopy for detection of anorectal pathology (92). Fluoroscopy and dynamic MRI can be used in situations involving severe multicompartmental prolapse or in which the severity of the symptoms is disproportionate to examination findings.
Anal Manometry
Anal manometry is used to determine maximum resting pressure, maximum squeeze pressure, rectal sensation and compliance, as well as the presence of an intact rectoanal inhibitory reflex. With disordered defecation, it can be used to diagnose Hirschsprung disease and anismus. The addition of surface EMG to document relaxation helps exclude anismus as a cause of obstructed defecation. Failure of the anal sphincter to relax with defecation and increased electrical activity of the EAS and puborectalis are seen in patients with anismus. In contrast, there should be no increase in the electrical activity measured by surface electrodes for patients with Hirschsprung disease. A rectal balloon expulsion test can also be of assistance in the evaluation of rectal emptying and may be valuable during physiotherapy for diagnosing dyssynergic defecation.
Colonoscopy and Proctoscopy
Standard gastrointestinal evaluation for patients with symptoms of disordered defecation should include a barium enema or colonoscopy to eliminate the possibility of colorectal malignancy. Proctoscopy should be included as part of the routine examination because it may reveal anorectal pathology.
Therapeutic Approach to Fecal Incontinence
Treatment of fecal incontinence should first focus on nonsurgical options, including dietary modification, medical therapy, and biofeedback. Any underlying systemic conditions or gastrointestinal disorders should be treated before initiating an extensive evaluation for other causes of fecal incontinence. If symptoms persist, further investigation should be undertaken. If the evaluation discloses an underlying EAS defect and conservative therapy has been unsuccessful, it is reasonable to proceed with surgical treatment.
Following is an overview of treatment options and the efficacy of each approach. The lack of consistent outcome measures makes it difficult to compare efficacy among treatments. Some studies base success on strict conformity with criteria for continence, but the results vary for continence of flatus, liquid, or solid stool. Other studies base success on more subjective criteria, such as improvement following treatment. Daily diaries can be maintained, but the results may be unreliable. Even if a validated symptom survey and quality-of-life scale are employed, few studies use the same outcome measure.
Nonsurgical Treatment
Nonsurgical management focuses on maximizing the continence mechanism through alteration of stool characteristics or behavioral modification. Stool consistency and volume can be manipulated by dietary and pharmacologic means to achieve passage of one to two well-formed stools per day. The rationale for this approach is that formed stool is easier to control than liquid stool. Additionally, behavior modification can be employed using bowel regimens that focus on the predictable elimination of feces. Physical therapy and biofeedback can also be useful for strengthening the continence mechanism.
Pharmacologic Approaches
Dietary Modification and Fiber
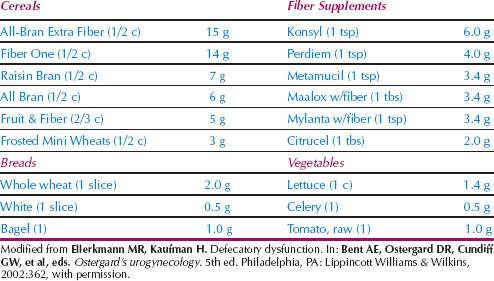
Dietary modification for treatment of fecal incontinence frequently involves avoidance of foods that precipitate loose stools and diarrhea. Common dietary irritants include spicy foods, coffee and other caffeinated beverages, beer and alcohol, and citrus fruits. Avoidance of dairy products or the addition of lactase dietary supplements is essential for those with lactose intolerance. The addition of fiber may improve fecal incontinence by functioning as a stool bulking agent to increase volume and density. The average individual in the United States consumes less than half the recommended daily fiber intake (25–35 g). Various fiber sources are listed in Table 28.9, with the highest content found in high-fiber cereals. It is difficult to consume the recommended daily amount from diet alone, and fiber supplements often are required. Although the increased stool volume and density helps many individuals maintain continence, excessive fiber with inadequate fluid intake may predispose elderly patients to fecal impaction.
Table 28.9 Fiber Sources
Constipating Agents
Constipating agents have the most value in patients with chronic loose stools or diarrhea. They can also help improve symptoms in patients with fecal frequency and urgency. Loperamide (Imodium) and diphenoxylate hydrochloride with atropine (Lomotil) are the most commonly used agents. Loperamide has been shown to prolong transit time and stimulate anal sphincter function. With either of these agents, careful titration is recommended to prevent the primary side effect of constipation. It is generally preferable to begin using 2 to 4 mg of loperamide daily and then titrate up to 4 mg three to four times per day. A 4-mg dose before meals has been shown to increase anal tone and improve continence (93). Lomotil is started at a dose of one to two tablets every day or every other day and titrated up to one to two tablets three to four times a day as needed. Caution should be exercised for patients taking other anticholinergic medications. Anticholinergic side effects include dry mouth, drowsiness, lightheadedness, and tachycardia. Codeine can also be used as a constipating agent. It should be used judiciously in those with chronic disorders and in elderly patients because of side effects common to narcotics, including addiction with prolonged usage and central nervous system and respiratory depression. A study of 82 geriatric patients documented the efficacy of pharmacologic treatment for fecal incontinence (94). Patients were treated based on the underlying cause. Those with fecal impaction received lactulose and enemas, whereas those with neurogenic fecal incontinence received codeine phosphate as a constipating agent and enemas. The rate of cure for fecal incontinence was 60% in the treatment group versus 32% for controls (P <.001).
Medications for Irritable Bowel Syndrome
Dietary treatment of IBS consists of avoiding foods that are associated with symptoms, including alcohol, caffeine, sorbitol, and foods that increase gas production. Although increased dietary fiber or fiber supplementation has been shown to improve the constipation-predominant form of this illness, fiber supplementation has little effect on the diarrhea variant associated with fecal incontinence. Pharmacologic therapy is directed toward the predominant symptom. Loperamide and Lomotil tend to be useful first-line agents for treating diarrhea. Tricyclic antidepressants improve abdominal discomfort and are also valuable in diarrhea-predominant patients because of their constipating effect. The serotonin type 3 (5HT3) antagonist alosetron (Lotronex) has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of severe diarrhea-predominant IBS refractory to treatment. It has shown improvement in global assessment measures, but its use is limited because of multiple isolated case reports of ischemic colitis. The recommended dose is 1 mg once or twice daily. It does not appear to be effective for the spastic-pain variant of IBS. Anticholinergics (dicyclomine, hyoscyamine) and antispasmodics (mebeverine, pinaverine[MB3]) are targeted at the pain and bloating symptoms but may also be useful for the diarrhea variant because of their constipating side effects. Studies comparing anticholinergic medications to placebo show inconclusive results with only modest benefits. Antispasmodic agents may also be of value and are available in many countries but are not approved for use in the United States. Currently, additional 5HT3 antagonists and 5HT4 antagonists are under development and are approved for use in Europe but not in the United States. Most studies are poorly designed and difficult to interpret because of a high placebo response rate that often exceeds 30% (95,96).
Behavioral Approaches
Biofeedback
Biofeedback can be an effective therapeutic modality provided patients are motivated and comprehend instructions. The two proposed mechanisms through which biofeedback improves fecal continence are afferent and efferent training. Afferent training focuses on improving sensation in the anorectal canal through recruitment of adjacent neurons to decrease the sensory threshold of volume stimulation. The goal of this training is to enhance and restore anal sensation and the rectoanal inhibitory reflex. Efferent training enhances and restores voluntary contraction of the EAS, which permits additional recruitment of motor units and stimulates muscle hypertrophy. These two methods of training can be performed independently but are often combined for additional therapeutic benefit. The most common training method uses an intrarectal balloon. The balloon acts to stimulate rectal distention and provide pressure feedback from coordinated or synchronized contraction of the pelvic floor muscles. Other techniques focus on strength training of the EAS alone using anal pressure feedback or EMG or afferent training alone using an intrarectal balloon without pelvic floor muscle contraction in response to the stimulus.
More than 35 studies have been done to evaluate the efficacy of biofeedback for treatment of fecal incontinence, and several excellent review articles and meta-analyses have determined the effects of individual treatments and predictors of patient response to treatment (97–99). The results of all of these studies uniformly agree that biofeedback and pelvic floor exercises improve fecal incontinence and have a role in clinical practice. They also agree that the existing literature is fraught with methodologic problems and lacks validated outcomes and controls. Thus it is difficult to compare directly the study results.
Biofeedback is an ideal first-line therapy because it offers an effective, minimally invasive treatment without any reported adverse events. Biofeedback also appears to provide a higher probability of successful outcome than standard medical care for treating functional fecal incontinence (67% vs. 36%, respectively, P <.001) (97).
A Cochrane review of biofeedback and exercises for treatment of fecal incontinence found only five randomized or quasi-randomized control trials that qualified for inclusion (100). The authors concluded that there is insufficient evidence to evaluate the efficacy of exercises and biofeedback for treatment of fecal incontinence. Specifically, they were not able to determine which patients were suitable for treatment nor which method of treatment was optimal. A meta-analysis of biofeedback techniques included a review of 13 studies using strength training alone, 4 studies with sensory training alone, and 18 with coordinated sensory and strength training (99). The authors found no advantages between coordinated training (67% improved) and strength training (70% improved). However, strength training using EMG appeared to be better than strength training with anal canal pressure biofeedback (74% vs. 64% improved, respectively, P <.04). The limitations of this study and the literature were acknowledged.
A large randomized control trial of biofeedback for fecal incontinence in 171 patients, divided into four treatment groups, showed no significant benefit when comparing standard care to similar care with the addition of biofeedback (54% vs. 53% improvement, respectively) (101). In all groups, there was a high median rating of change of symptoms and median satisfaction with benefits relatively maintained at 1-year follow-up. All groups displayed improvement in the validated symptom surveys and quality-of-life measures as well as in anal sphincter function. The authors concluded that interactions with the therapist, patient education, and development of better coping strategies seem to be the most important factors for improvement rather than pelvic muscle exercises or biofeedback. Additional benefit may be derived with augmented biofeedback using electrical stimulation (102).
There are no clear indicators to predict which patients will benefit from biofeedback. Potential factors include age, duration and severity of incontinence, prior treatments or surgery, and severity of neurologic or physical damage. Controversy exists as to whether response to biofeedback is dependent on the presence of a structurally intact anal sphincter or normal pudendal nerve function (103–106). Similarly, there is insufficient evidence that electrical stimulation improves fecal incontinence (107). There is an obvious need for well-designed control trials using validated symptom surveys and quality-of-life instruments. More objective measures are desirable, and studies should carefully document duration of treatment and length of follow-up.
Bowel Regimens
The goal of bowel regimens is to achieve predictable elimination of feces. This can be accomplished by using the gastrocolic reflex as well as by dietary and pharmacologic means. Defecation immediately following meals involves the physiologic response of the gastrocolic reflex to facilitate predictable emptying. The strength of the gastrocolic reflex varies among individuals and may be hypoactive or hyperactive with certain systemic disorders, such as diabetes and multiple sclerosis. This technique can be especially useful in the morning to give the individual freedom from fecal incontinence throughout the day. The use of suppositories or enemas in the morning or at night in conjunction with the gastrocolic reflex may provide further relief of daytime symptoms. The goal is to leave the rectum empty between evacuations. Enema use, typically once or twice daily, should be titrated to the patient’s baseline colonic activity. Regular toileting in elderly patients in nursing homes can improve fecal incontinence caused by overflow incontinence from fecal impaction. The use of cone-tip colostomy-irrigation catheters is reserved for patients in whom other therapeutic modalities have failed. These catheters avoid the risk of rectal perforation and provide a dam to prevent efflux of the irrigating solutions (108).
Surgical Treatment
In general, surgical treatment should be employed after conservative measures have failed. Although there may be exceptions to this principle, most surgeons follow this recommendation because of the poor long-term outcomes and high complication rates with surgery for fecal incontinence.
Overlapping Sphincteroplasty
Overlapping sphincteroplasty is the procedure of choice for fecal incontinence caused by a disrupted anal sphincter. Most authorities believe that an overlapping technique is superior to an end-to-end repair, although there are few direct comparisons in the literature. The rationale for the overlapping technique is that a more secure repair can be accomplished by placing sutures through the scarred connective tissue rather than the sphincter muscle. Sutures should be less likely to tear through or pull out of connective tissue than muscle. Therefore, the key component of the overlapping technique is preservation of the scarred ends of the ruptured EAS for suture placement.
Technique
The initial step involves wide mobilization of the ruptured EAS without excision of the scarred ends of the sphincter. This is accomplished through an inverted semilunar perineal incision or a transverse incision near the posterior vaginal fourchette with inferiolateral extension. The latter incision facilitates repair in patients with damage to the rectovaginal septum attachment to the perineal body. Patients with EAS defects have either a band of intervening fibrous scar tissue between the viable muscular ends of sphincter or a complete separation with scar tissue present only on the ruptured ends of the sphincter. In the presence of complete separation of scar tissue, perineal body reconstruction usually is indicated at the time of repair to restore normal anatomy. A Peña muscle stimulator aids in identification of the distal ends of the EAS and differentiates viable muscle tissue from scar tissue. The stimulator can be used to outline the sphincter before incision as well as during the dissection. It is important to apprise the anesthesiologist of the stimulator usage so that paralytic agents are avoided.
Excessive lateral dissection of the EAS past the 3 and 9 o’clock positions should be avoided as this is where the inferior rectal branches of the pudendal nerve innervate the EAS. Moderate bleeding often is encountered during this dissection, and the use of needlepoint electrocautery can maximize hemostasis. Controversy exists regarding the need to separate the EAS and IAS before repair. Identification of the intersphincteric groove facilitates dissection of the EAS. Dissection in this plane is relatively simple and avoids damage to either sphincter. Defects in the IAS can be more difficult to visualize because this muscle is intimately associated with the rectal mucosa. Examination with a finger in the anal canal is often helpful.
The reconstruction begins with repair of an existing IAS defect using a 3-0 delayed absorbable monofilament suture. Next, the EAS defect is repaired with the primary goal of overlapping at least 2 to 3 cm to ensure adequate bulk of sphincter muscle encircling the anal canal. The EAS is overlapped using three to four mattress sutures of 2-0 delayed absorbable monofilament suture through the distal scar tissue. Once the sutures are tied, there should be resistance palpable with placement of a finger in the anal canal. Copious irrigation is performed throughout the procedure. Following sphincter repair, a perineal body reconstruction and rectocele repair should be undertaken, if indicated, to maximize the normal continence mechanism. Finally, the perineal skin is closed with interrupted absorbable monofilament sutures. Closure frequently requires modification of the initial incision because of changes in the perineal architecture that result from the repair. The most common approach is an inverted Y-shaped closure of the incision (Fig. 28.4).
Figure 28.4 Overlapping sphincteroplasty procedure. A: Inverted semilunar perineal incision with the distal ends of the external sphincter outlined using the Peña muscle stimulator. B: The external sphincter has been dissected, the scar divided in the midline, and the internal sphincter repaired. C: The external sphincter is overlapped using three mattress sutures of 2–0 delayed absorbable monofilament suture through the distal scar tissue. D: The sutures are tied. E: The skin is closed.