Human steroidogenic and steroid-inactivating enzymes in peripheral intracrine tissues. 4-dione, androstenedione; A-dione, 5α-androstane-3,17-dione; ADT, androsterone; epi-ADT, epiandrosterone; E1, estrone; E1-S, estrone sulfate; E2, 17β-estradiol; E2-S, estradiol sulfate; 5-diol, androst-5-ene-3α, 17β-diol; HSD, hydroxysteroid dehydrogenase; testo, testosterone; RoDH-1, Ro dehydrogenase 1; ER, estrogen receptor; AR, androgen receptor; UGT2B28 family (including UGT2B7, UGT2B15, and UGT2B17), uridine glucuronosyl transferase 2B28; Sult2B1, sulfotransferase 2B1; UGT1A1, uridine glucuronosyl transferase 1A1.
DHEA becomes the practically exclusive source of sex steroids (both androgens and estrogens) after menopause
At menopause, the ovary becomes completely depleted of estrogen-producing follicles and the secretion of E2 by the ovary into the circulation practically ceases. The consequence is that serum E2 decreases from values of at least 80 pg/mL in premenopausal women to an average of 4.2 pg/mL after menopause, with 95% of women having serum E2 concentrations below 9.2 pg/mL [4]. These low biologically inactive E2 concentrations avoid stimulation of the endometrium, which shows atrophy in all women after menopause. This extremely positive aspect of menopause, namely the protection from rapid appearance of endometrial cancer, has provided a decisive factor for evolutional forces to choose the lineage of women having menopause and non-estrogen-secreting ovaries after the reproductive years. For serum testosterone, on the other hand, no significant change [33,34] or a small 15% decline [35] has been reported between pre- and postmenopause. In fact, as mentioned above, the serum levels of testosterone in postmenopausal women are similar to those of castrated men [3], while in intact men, serum testosterone is about 25-fold higher due to the direct secretion of testosterone in the bloodstream by the testicles.
While the cessation of reproduction and the arrest of ovarian estrogen secretion long before the end of life are essential characteristics of menopause, it is equally important to recognize that in the hypothetically complete absence of sex steroids, the life of women after menopause would have been of poor quality and likely to be seriously shortened, since practically all bodily functions are modulated, to various degrees, by estrogens and/or androgens [13,36]. In fact, in the absence of the estrogens and androgens made specifically in each cell type of each tissue from circulating DHEA by intracrine mechanisms, the problems presently affecting women at menopause, especially osteoporosis and fractures, hot flushes, muscle loss, type 2 diabetes, vulvovaginal atrophy, sexual dysfunction, memory loss, cognition loss, and possibly Alzheimer’s disease, would be much more serious than presently observed with a likely significant reduction in quality of life and even in lifespan. In other words, while serum E2 must remain at sub-threshold or biologically inactive concentrations in the bloodstream after menopause in order to avoid endometrial stimulation and the risk of endometrial cancer, the normal functioning of most of the other peripheral tissues requires intracellular biologically active concentrations of estrogens and/or androgens.
Medical research, however, has concentrated almost exclusively on the arrest of E2 and progesterone secretion by the ovary and how to replace estrogens. One practically never envisaged that the arrest of secretion of E2 by the ovary into the circulation at menopause could be a positive factor resulting from elimination of the risk of endometrial hyperplasia and carcinoma, instead of being exclusively a negative phenomenon requiring estrogen replacement. As mentioned above, in the presence of the steroid-forming and steroid-inactivating enzymes specifically expressed in each cell type of each human peripheral tissue (Figure 6.1), coupled with the availability of the precursor DHEA (Figure 6.2), all the elements were in place for delivery of the required amounts of sex steroids to each cell in each tissue, and thus minimize the negative impact of the arrest of E2 secretion by the ovary at menopause. It does not mean that the arrest of E2 secretion by the ovary has no causative role in the menopausal symptoms, but one has to consider that evolution/nature has designed an alternative to E2 replacement, namely DHEA, a precursor inactive by itself, in order to distribute the sex steroids needed in a tissue-specific manner in the whole organism without biologically significant release of the active sex steroids in the circulation.
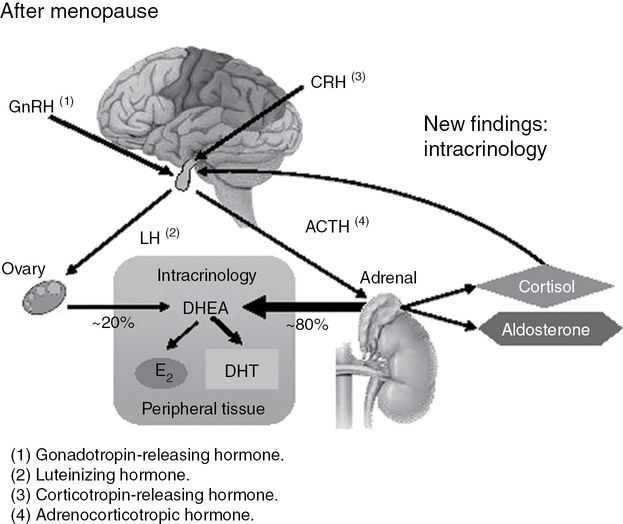
Schematic representation of dehydroepiandrosterone (DHEA) acting as the unique source of sex steroids in women after menopause. Approximately 80% of circulating DHEA is of adrenal origin while about 20% is released from the ovary [4]. Accordingly, after menopause, all androgens and all estrogens are made locally from DHEA in peripheral target tissues. The amount of sex steroids made depends upon the level of the steroid-forming enzymes specifically expressed in each cell in each tissue. GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; CRH, corticotropin releasing hormone; ACTH, adrenocorticotropic hormone; E2, 17β-estradiol; DHT, dihydrotestosterone. From Labrie et al. [4].
The secretion of DHEA, however, markedly decreases with age, with an average 60% loss already observed at the time of menopause [1,21,37,38]. The marked reduction in the secretion of DHEA with age [38] results in a parallel fall in the formation and availability of androgens and estrogens in peripheral target tissues, a situation believed to be associated with the series of medical problems of menopause mentioned above [32].
It is very important to mention that an essential aspect of intracrinology is the fact that the active sex steroids are not only made locally, but that they are also inactivated locally in the same cells where synthesis takes place. In fact, the sex steroids made from DHEA in peripheral tissues are essentially released outside the cells as inactive compounds. As illustrated in Figure 6.3, DHEA of either adrenal, ovarian, or exogenous (for example, tablet or cream) origin is distributed by the general circulation to all tissues indiscriminately. The transformation of DHEA into estrogens/androgens, however, is tissue specific, ranging from none in the endometrium to various levels in the other tissues of the human body. Most importantly, approximately 95% of the active estrogens and androgens made are inactivated locally before being released in the blood as inactive metabolites, thus avoiding inappropriate exposure of the other tissues [13] (Figure 6.3).
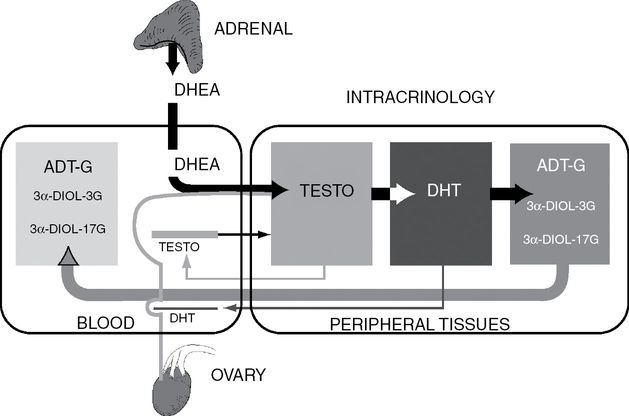
Schematic representation of the role of the precursor DHEA of both adrenal (~80%) and ovarian (~20%) origins in total androgenic activity in postmenopausal women. A very small proportion of the active androgens testosterone and DHT made intracellularly by the steroidogenic enzymes of the intracrine pathway diffuse into the circulation, thus avoiding systemic effect. The height of the bars is proportional to the concentration of each steroid. For androgens, the mechanisms of intracrinology [2] make it possible to keep intracellularly the formation and inactivation of androgens with only some leakage at very low levels in the circulation. In the circulation, the biologically significant and representative parameters are the precursor DHEA and the glucuronidated metabolites of androgens (ADT-G and 3α-diol-3G and 3α-diol-17G).
Variable/rate-limiting circulating levels of DHEA and indirect role of the ovary in androgen formation
Because, as indicated above, after menopause, circulating DHEA is the only source of sex steroids made locally in peripheral tissues by the process of intracrinology [2,15,25,39], it seems important to examine the interindividual variability in the serum levels of DHEA and its metabolites. It is also of interest to investigate the potential role of the postmenopausal ovary in the direct secretion of sex steroids by comparing the steroid levels in intact and ovariectomized (OVX) women using MS-based assays [4, 12–15]. The data obtained show a high interindividual variability in DHEA and all its metabolites, with no evidence of a direct secretion of estrogens or androgens by the postmenopausal ovary, which does seem, however, to contribute approximately 20% of total circulating DHEA, and consequently, around 20% of total sex steroids after menopause in this age group[4].
The serum DHEA levels in intact 46- to 74-year-old postmenopausal women were measured at 2.03 ± 1.33 ng/mL (mean ± SD) (5th–95th centiles, 0.55–4.34 ng/mL) with a median value of 1.73 ng/mL, whereas an 18.2% lower mean value (1.66 ± 1.04 ng/mL) was found in OVX women of similar age (42–74 years old) (P = 0.0102). Compared with 30- to 39-year-old premenopausal women having DHEA measured at 4.47 ± 2.19 ng/mL, the mean serum DHEA concentration was 55% lower in intact postmenopausal women and 63% lower in OVX postmenopausal women, thus indicating the contribution of the ovary [4].
Similar observations were made for serum dehydroepiandrosterone sulfate (DHEA-S). The mean serum DHEA-S levels were 19.0% lower in OVX women compared with intact postmenopausal women, with values of 0.63 ± 0.41 μg/mL (5th–95th centiles = 0.15–1.38 μg/mL) in intact and 0.51 ± 0.39 μg/mL (5th–95th centiles = 0.13–1.00 μg/mL) in OVX postmenopausal women (P = 0.0181). Serum testosterone, on the other hand, was 21.4% lower in OVX compared with intact women. In fact, serum testosterone decreased from a mean value of 0.14 ± 0.08 ng/mL in intact women to 0.11 ± 0.05 ng/mL in OVX subjects (P <0.0001) [4].
When calculations are made to compensate for the higher serum DHEA levels observed in intact women compared with OVX women, no significant contribution of androgens by the postmenopausal ovary independently from DHEA could be found. The 18.2% lower serum DHEA observed in OVX women compared with intact women can well explain the 21.4% lower serum testosterone and the 16.6% lower serum E2 in OVX women compared with intact postmenopausal women, thus leaving no significant role for a direct contribution of the postmenopausal ovary to circulating testosterone and E2 [4].
Because the lower serum DHEA levels found in OVX women can explain the lower serum concentrations of all measured sex steroids and metabolites, it can be logically concluded that the postmenopausal ovary does not secrete significant amounts of estrogens or androgens directly into the circulation. Sex steroid secretion by the postmenopausal ovary is a controversial area, especially in regard to immuno-based assays of testosterone. Most studies, however, have reported that the contribution of the postmenopausal ovary to circulating active androgens is not significant [40–45].
The data summarized above indicate that the persistence of low testosterone concentrations in the blood of postmenopausal women is not only “largely due,” [33,46,47] but is rather entirely due to the peripheral conversion of DHEA into testosterone, which leaks in small amounts into the extracellular space and then into the general circulation. Such a possibility is supported by the finding that the postmenopausal ovary has persistent, although much reduced as compared with the corpus luteum of the premenopausal ovary, transcript levels of the cholesterol transport protein steroidogenic acute regulatory cholesterol side chain cleavage enzyme (CYP11A) and 17α-hydroxylase-17,20 lyase enzyme (CYP17), with extremely low levels of type 2 3βhydroxysteroid dehydrogenase and aromatase (CYP19) [45]. This possibility is further supported by the presence of CYP17 in the stroma of the postmenopausal ovary, whereas the type 2 hydroxysteroid dehydrogenase protein is absent, thus favoring the formation of DHEA [45]. From the serum levels of testosterone and the intraprostatic concentrations of dihydrotestosterone (DHT) observed in men before and after castration [3], it could be estimated that only about 5% of the intracellular testosterone diffuses into the general circulation, a situation which is likely to be similar in the peripheral tissues of women.
The 18%, but parallel lower serum levels of DHEA and all its metabolites, found in ovariectomized women, including E2 and testosterone, strongly suggest that the postmenopausal ovary secretes approximately 20% of total DHEA in the 42- to 72-year-old age group of intact postmenopausal women, with no significant E2 or testosterone direct secretion by the ovary into the general circulation. There is no reason to believe that the DHEA of ovarian origin is not submitted to the same intracrine mechanisms as the DHEA of adrenal origin.
The serum levels of androgen metabolites, but not of testosterone, as markers of androgen exposure in women
It is a well-recognized issue that the long series of case-control and prospective cohort studies, which analyzed a potential correlation between serum testosterone and the incidence of obesity, insulin resistance, sexual dysfunction, or other clinical problems in women, generally yielded contradictory results [37,48–56]. This lack of consistency between the serum levels of testosterone and the observed effect of exogenous androgens has raised serious doubts about the validity of measurements of total as well as free serum testosterone as markers of androgenic activity in women.
As described above, the lack of correlation between serum testosterone and clinical parameters believed to be under androgen control can be best explained by the above-summarized data showing that practically all androgens in women are made locally in peripheral target tissues from the inactive precursor DHEA of adrenal and ovarian origins [2,34,57]. Since the androgens made locally do not originate from the very low circulating testosterone, one could reasonably conclude that measurement of serum testosterone is of highly questionable clinical significance. In fact, as indicated above, the androgens testosterone and DHT made in peripheral tissues from circulating DHEA exert their action locally in the same cells where synthesis takes place, with only minimal and variable release as active androgens in the circulation. Testosterone and DHT are then inactivated in the same cells into water-soluble glucuronide and sulfate derivatives, which diffuse quantitatively into the general circulation, where they can be measured before their elimination by the kidneys (Figure 6.3) [13,15].
Considering the major importance for clinicians and women to have access to a valid and reliable marker of androgenic activity in order to assess with confidence the role of androgens in a series of problems particularly frequent after menopause, namely type 2 diabetes, obesity and arteriosclerosis (metabolic syndrome), sexual dysfunction, osteoporosis, breast cancer, skin atrophy as well as loss of muscular strength, physical fitness, and well-being, we have used LCMS/MS and GC-MS to measure nine androgens, their precursors, and metabolites in 377 postmenopausal women in good health aged 55–65 years in order to analyze the correlation between serum testosterone and the true markers of the total pool of androgens, namely the glucuronide derivatives of androsterone (ADT) and androstane-3α, 17β-diol (3α-diol), the obligatory route of elimination of androgens. We have also compared the results with data obtained in 47 30- to 35-year-old normally cycling women (Table 6.1).
Group | Value | DHEA (ng/mL) | 5-Diol (ng/mL) | Testo (ng/mL) | DHT (ng/mL) | E1 (pg/mL) | E2 (pg/mL) |
|---|---|---|---|---|---|---|---|
55- to 65-year-old postmenopausal women (n = 377) | Mean | 1.95 | 0.27 | 0.14 | 0.04 | 17.78 | 4.17 |
SD | 1.18 | 0.15 | 0.07 | 0.03 | 10.04 | 3.29 | |
Median | 1.72 | 0.25 | 0.13 | 0.03 | 15.58 | 3.44 | |
5th–95th centiles | 0.56–3.99 | 0.1–0.54 | 0.06–0.26 | 0.01–0.07 | 7.57-34.8 | 1.0–9.27 | |
(Min–max) | (0.1–11.2) | (0.1–0.85) | (0.03–0.57) | (0.01–0.29) | (4.0–103) | (1.0–30.0) | |
30- to 35-year-old premenopausal women (n = 47) | Mean | 4.47 | 0.49 | 0.18 | 0.07 | 54.0 | 82.0 |
SD | 2.19 | 0.20 | 0.07 | 0.03 | 23.3 | 42.2 | |
Median | 4.14 | 0.44 | 0.17 | 0.07 | 49.5 | 71.4 | |
5th–95th centiles | 1.53–9.14 | 0.25–0.84 | 0.06–0.31 | 0.03–0.14 | 23.7–87.5 | 22.0–160 | |
(Min–max) | (1.41–10.4) | (0.25–0.96) | (0.05–0.32) | (0.03–0.17) | (18.3–123) | (17.7–181) | |
Group | Value | E1-S (ng/mL) | DHEA-S (μg/mL) | 4-Dione (ng/mL) | ADT-G (ng/mL) | 3α-Diol-3G (ng/mL) | 3α-Diol-17G (ng/mL) |
55- to 65-year-old postmenopausal women (n = 377) | Mean | 0.22 | 0.59 | 0.40 | 15.8 | 0.64 | 0.57 |
SD | 0.21 | 0.36 | 0.18 | 12.5 | 0.52 | 0.47 | |
Median | 0.17 | 0.55 | 0.37 | 13.1 | 0.55 | 0.25 | |
5th–95th centiles | 0.04–0.59 | 0.15–1.24 | 0.17–0.71 | 3.27–41.7 | 0.25–1.69 | 0.25–1.54 | |
(Min–max) | (0.04–2.00) | (0.04–2.44) | (0.10–1.37) | (1.00–79.4) | (0.25–3.48) | (0.25–3.56) | |
30- to 35-year-old premenopausal women (n = 47) | Mean | 1.19 | 1.27 | 0.96 | 40.2 | 1.21 | 1.43 |
SD | 0.93 | 0.62 | 0.35 | 29.3 | 0.83 | 0.93 | |
Median | 0.87 | 1.04 | 0.92 | 31.6 | 1.06 | 1.35 | |
5th–95th centiles | 0.31–3.50 | 0.56–2.65 | 0.45–1.64 | 12.2–118 | 0.25–2.78 | 0.25–2.56 | |
(Min–max) | (0.21–4.40) | (0.45–2.71) | (0.31–1.77) | (6.86–133) | (0.25–4.33) | (0.25–5.71) |
Serum concentrations measured in 30- to 35-year-old premenopausal (n = 47) and 55- to 65-year-old postmenopausal (n = 377) women are indicated (Labrie, Bélanger et al. 2006 [1]).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


