Representation of the role of ovarian and adrenal sources of sex steroids in women. After menopause, the secretion of estradiol by the ovaries ceases and almost all of the sex steroids are made locally in peripheral target intracrine tissues including the skin. LH, luteinizing hormone; LHRH, luteinizing hormone-releasing hormone; ACTH, adrenocorticotropic hormone. Adapted from “Intracrinology and the skin,” by F. Labrie, V. Luu-The, C. Labrie, G. Pelletier, and M. El-Alfy, 2000, Hormone Res, 54, p. 219.
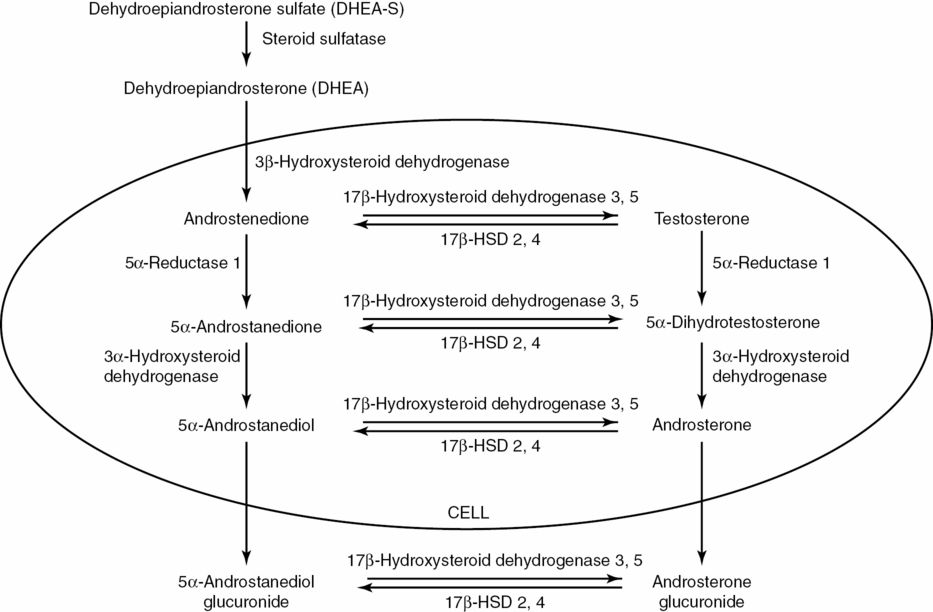
Pathways of cutaneous androgen metabolism and the converting enzymes. Adapted from “Cutaneous androgen metabolism: basic research and clinical perspectives,” by W. Chen, D. Thiboutot, and C. C. Zouboulis, 2002, J Invest Dermatol, 119, p. 993.
Although the skin is not the major site of androgen production, the circulating prohormones DHEA and androstenedione can be converted into testosterone and DHT in sebocytes, sweat glands, and dermal papilla cells [4]. Consequently, these potent androgens can impact dermal functioning. Sweat glands and sebaceous glands account for the vast majority of androgen metabolism in the skin [5]. The peripheral organs produce 100% of the active sex steroids in postmenopausal women [6] (Figure 8.1). However, it remains unclear what proportion of the androgens made in the skin can be attributed to de novo synthesis from epidermally formed cholesterol versus cutaneous conversion of circulating prohormones into potent androgens [7].
Androgen receptor
The effects of testosterone and DHT are mediated through a nuclear receptor called the androgen receptor (AR). Testosterone and DHT are both able to bind to AR, but DHT has a tenfold higher affinity for AR compared with testosterone [8]. Located in the cytoplasm, AR consists of multiple protein subunits, including the heat shock proteins HSP90, HSP70, and HSP56. Upon binding its androgen ligand, AR dissociates from the heat shock proteins, exposing a nuclear translocation signal that allows the androgen–AR complex to move from the cytoplasm into the nucleus. Here, the complex acts as a transcription factor, binding to the promoters of androgen-regulated genes and influencing their expression [4].
The AR gene is located on the X chromosome and expressed in epidermal and follicular keratinocytes, sebocytes, sweat gland cells, dermal papilla cells, dermal fibroblasts, endothelial cells, and genital melanocytes [9]. AR expression has been shown to be upregulated in genital skin fibroblasts and sebocytes [10]. In addition to its activation by androgen binding, AR function can be further regulated through binding of its N-terminal domain or ligand-binding domain by more than 200 identified co-regulators, including transcription factors, kinases, chaperones, cytoskeletal proteins, and histone modifiers [11]. The complexity of its regulation reflects the role of AR as an important synthesizer and convergence point of cellular signaling and function.
Androgen metabolism in the skin
In contrast to classic steroidogenic organs, such as the gonads and adrenal glands, the skin is considered a peripheral endocrine organ that both produces and is targeted by androgens [12]. The local concentration of each androgen depends on the expression level of each androgen-synthesizing enzyme in a particular cell type, especially the sebaceous glands and sweat glands [13] (Figure 8.2). The skin is capable of producing cholesterol, but expresses very little cytochrome P450c17, the enzyme necessary for synthesizing DHEA and androstenedione. However, sebocytes, sweat glands, and dermal papilla cells in the skin do possess adequate amounts of the enzymes needed to convert DHEA and androstenedione (and possibly DHEA-S) into the more potent testosterone and DHT [12].
Five key enzymes play a role in the cutaneous metabolism of androgens. First, steroid sulfatase hydrolyzes DHEA-S to DHEA. Next, 3β-hydroxysteroid dehydrogenase (3β-HSD) converts DHEA to androstenedione in sebaceous glands. Androstenedione is then activated by converting it to testosterone via isoforms 3 and 5 of androgenic 17β-hydroxysteroid dehydrogenase (17β-HSD). 17β-HSD types 2 and 4 catalyze the reverse oxidation reaction that inactivates testosterone into androstenedione. 5α-Reductase irreversibly converts testosterone to DHT, particularly in sebaceous and sweat glands and to a lesser extent in epidermal cells and hair follicles in the skin [5]. There are two isoforms of 5α-reductase: type I dominates in the skin while type II occurs mainly in beard hair follicles [8]. Finally, 3α-hydroxysteroid dehydrogenase (3α-HSD) breaks down active androgens into compounds that no longer bind AR [13]. Alternatively, aromatase can convert testosterone and androstenedione to estrogens in sebaceous glands, hair follicles, and dermal papilla cells [11].
Effects on the sebaceous gland
Sebaceous gland enlargement and sebum production both rely on androgens [5]. AR has been identified in basal and differentiating sebocytes, indicating that androgens contribute to cell proliferation and lipogenesis [14]. Androgens are known to stimulate sebocyte proliferation, especially in facial sebocytes. However, androgens alone are unable to modify sebocyte differentiation and require peroxisome proliferator-activated receptor (PPAR) ligands in order to stimulate sebocytes to differentiate [15].
Effects on the hair follicle
Androgens influence hair growth by acting through type 2 5α-reductase and AR in dermal papilla cells. By releasing growth factors that act in paracrine fashion on other follicle cells, dermal papilla cells mediate the growth-stimulating effect of androgens [16]. Androgens promote the enlargement of hair follicles in some androgen-dependent areas, such as the axillary and pubic regions, but cause miniaturization and shortage of hair in other areas, such as the scalp in susceptible individuals [3]. The differential response of dermal papilla cells to androgens may depend on genetically determined differences. For example, AR mRNA has been shown to be expressed at high levels in dermal papilla cells found in axillary hair, but at low levels in dermal papilla from occipital scalp hair [16].
Effects on the sweat gland
Given that at puberty males sweat at a higher rate than females in similar situations, androgens are thought to stimulate perspiration [17]. Over half of the skin’s 5α-reductase activity is present in sweat glands; moreover, sweat glands express AR and contain the enzymes required to convert DHEA to DHT. However, androgens are not thought to regulate the secretion rate of sweat glands directly, because androgen treatment has failed to stimulate sweat production in women and antiandrogen therapy has not decreased sweat production in men [2]. Rather than stimulating or maintaining the function of sweat glands, androgens likely exert their effect by initiating the factors required for differential sweat secretion between the sexes during puberty. Androgens are thought to influence the differentiation of apoeccrine sweat glands, which have a sevenfold higher secretory rate in response to similar innervation [18]. In addition, studies have reported that regardless of gender, apocrine glands of individuals with excessive or abnormal odor are typical target organs of androgens and contain predominantly type I 5α-reductase [19].
Human axillary odor results from a mixture of volatile organic compounds, including the steroids androstenol and androstenone, which arrive on the skin surface in apocrine secretions [20]. Variants in the ABCC11 gene, which encodes an ATP-dependent efflux pump expressed in apocrine sweat glands, is an important determinant of axillary odor. Individuals who are homozygous for a single-nucleotide polymorphism (SNP) (538G>A) were found to have a significantly lower amount of axillary odorants than either those who were heterozygous for this SNP or those who had the wild-type gene. The 538G>A SNP is predominant in Asians and rare in Africans and Europeans [20].
Further effects on skin
The skin of adult males has been shown to be thicker and drier than that of adult females. This difference is due in part to the epidermal hyperplasia and suppressed epidermal barrier function that results from androgens [21]. Furthermore, androgens are thought to inhibit cutaneous wound healing through binding to AR and modulating inflammatory responses [4]. The effect of androgens on wound healing remains an active area of research.
Pathogenesis and hormonally mediated treatment of androgen-related skin disorders
Acne vulgaris
Pathogenesis
Acne vulgaris is a disease of pilosebaceous follicles that involves follicular hyperkeratinization, excessive sebum production, Propionibacterium acnes (P. acnes) in the follicle, and excessive inflammation. Androgens contribute to the development of acne by promoting the growth and secretory function of sebaceous glands. The crucial role of androgens in the pathophysiology of acne (Figure 8.3) has long been confirmed. Clinical evidence includes the close correlation between the onset of acne in prepubertal children and the adrenarcheal rise in circulating levels of DHEA-S, acne formation in children with virilizing tumors or congenital adrenal hyperplasia, hyperandrogenism in women with sudden exacerbation of acne, acne induction by systemic or topical steroid use, and positive associations between serum androgen levels and acne lesion counts [22]. Furthermore, experimental studies have shown that sebaceous glands contain most of the steroidogenic enzymes needed for the conversion of DHEA and DHEA-S into testosterone and DHT [7], while immunohistochemistry and biochemical binding assays have shown that AR is present in the epithelial cells of sebaceous glands [23].
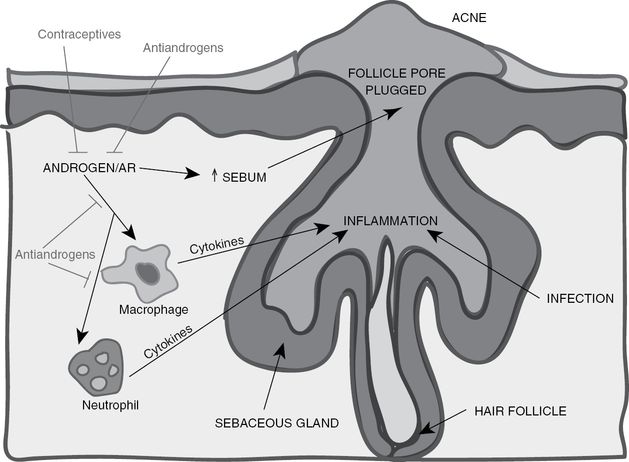
The role of androgens/AR in acne formation and progression. Acne formation results from excessive sebum formation accompanied by excessive inflammation and infections in the hair follicle. Macrophages and neutrophils are recruited to the inflamed follicles and secrete cytokines and other factors that promote inflammation and infection clearance. However, the inflammatory response also damages the normal tissues in the follicles. AR can promote the inflammatory response mediated by macrophages and neutrophils as well as directly promote sebum production that plugs the follicle pore. Current treatments for acne include antiandrogens and contraceptives, which reduce androgen levels, thereby reducing sebum production and suppressing the function of macrophages and neutrophils to dampen the inflammatory response. Adapted from “The role of androgen and androgen receptor in skin-related disorders,” by J. J. Lai, P. Chang, K. P. Lai, L. Chen, and C. Chang, 2012, Arch Dermatol Res, 304, p. 504.
The biological mechanism by which androgens stimulate sebocyte activity in acne is not fully understood. One possibility is that AR might enhance the activities of fibroblast growth factor receptor 2 (FGFR2), which previous studies have shown to be important for the development and homeostasis of sebaceous glands [24]. Another plausible mechanism is AR might increase lipogenesis in sebocytes by upregulating the expression of sterol-regulatory element-binding proteins (SREBPs). Androgens may also influence the activity of insulin-like growth factor-1 (IGF-1), which has been found to be able to induce SREBP-1 expression and thereby stimulate lipogenesis in sebocytes [25]. Finally, other studies in animal models have suggested that the androgen–AR complex may enhance the inflammatory responses of neutrophils and macrophages, and as a result, androgens not only promote sebaceous gland activity, but also contribute to the inflammation that leads to acne formation and progression [26]. Further research is needed to determine the exact mechanism of androgen-induced sebocyte activity.
Diagnosis
Evaluation of acne vulgaris requires particular attention to endocrine function. In addition to a review of cosmetic use for comedogenic products and medication history for acne-inducing drugs, such as glucocorticoids, phenytoin, and epidermal growth factor receptor (EGFR) inhibitors, the skin should be carefully examined for the type and location of lesions. Although acne is a common condition, a variety of disorders should be considered in the differential diagnosis. Rosacea, which is characterized by erythema, telangiectases, and papules or pustules on the central face, is distinguished from acne vulgaris, which is characterized by the presence of comedones and the absence of telangiectases [27]. Perioral dermatitis, which manifests as small, grouped, erythematous papules in a perioral (or occasionally perinasal or periorbital) distribution, has a rim of spared skin around the vermilion border of the lip. Sebaceous hyperplasia, or visible enlargement of sebaceous glands, commonly occurs in adults with a history of oily skin and appear as umbilicated yellowish papules usually on the forehead and cheeks. Staphylococcal, eosinophilic, or pseudomonal folliculitis may resemble inflammatory acne, but comedones are absent and lesions are monomorphous, unlike the polymorphous lesion in different developmental stages that are typical of acne [27]. Keratosis pilaris, which results from plugging of keratotic follicles, presents as small follicular papules on the face and extensor surfaces of the upper arms or thighs with or without erythema. Favre–Racouchot syndrome caused by sun damage is seen in middle-aged or older adults, who have open and closed comedones in areas of photodamage, often the lateral upper cheeks.
Treatment
Hormonal therapy is not usually the first option for treatment of acne vulgaris. However, a significant proportion of acne patients, anywhere between 30% and 80%, have been shown to display varying degrees of hyperandrogenemia [28]. A positive correlation between acne severity and markers of androgenicity was not found, but it is still possible to treat hyperandrogenemic patients with therapies that lower serum androgen levels or inhibit the action of androgens on sebaceous glands [4]. In female acne patients, oral contraceptives decrease free testosterone levels by 40–50% by suppressing luteinizing hormone, reduce androgen bioavailability by increasing the level of androgen-binding protein (sex hormone-binding globulin), and prevent the conversion of free testosterone to DHT by blocking AR and inhibiting 5α-reductase activity [29]. Flutamide, a nonsteroidal antiandrogen that prevents binding of DHT to AR, has been studied mostly in patients with hirsutism and has been shown to produce satisfactory results in treating both acne and hirsutism in these patients [30]. Topical inocoterone, which reduced the size of sebaceous glands in animal experiments, did not significantly improve acne in clinical studies [31]; however, inocoterone does lower inflammation in acne lesions, most likely by suppressing AR function. In contrast, the antiandrogen cyproterone failed to suppress testosterone-induced proliferation of sebocytes and keratinocytes in vitro, but oral and topical cyproterone acetate treatments have been shown to improve acne lesions [4]. Other inhibitors of AR, such as spironolactone, achieve some level of therapeutic effect on acne, but are limited by their significant adverse effects on menstruation, liver function, and pregnancy. Isotretinoin, an effective treatment for acne that suppresses sebum production, has been shown to modify AR signaling [32].
Androgenetic alopecia
Pathogenesis
Androgenetic alopecia is the most common type of hair loss, affecting 30–40% of adult men and women [33]. The hallmark of androgenetic alopecia is follicular miniaturization, the process by which the hair growth phase (anagen) is shortened, resulting in the production of a shorter, thinner hair shaft. As follicles are miniaturized, new hair growth continues, but becomes shorter and thinner. Follicular miniaturization is a hormonally mediated process that occurs at the level of the follicle. Although androgens normally stimulate hair production in many sites of the body, they have an opposite effect in the scalp: androgens suppress hair growth in susceptible areas of the frontal and vertex scalp. In the hair follicle, testosterone is converted to DHT, which is found at increasing concentrations in the balding scalp. DHT binds to AR in susceptible follicles, and the hormone-receptor complex moves into the nucleus, where it activates genes responsible for the transformation of large, terminal follicles into miniaturized follicles. As evidence for the role of AR in androgenetic alopecia, individuals lacking functional ARs do not experience balding, and AR expression levels were found to be elevated in frontal and vertex regions, but normal in the parietal and occipital regions of the scalps of patients afflicted with androgenetic alopecia [34]. The AR gene is the only risk gene for androgenetic alopecia confirmed to date, and AR gene variant(s) are generally accepted to be the primary culprit for the onset and development of androgenetic alopecia via abnormal expression of AR protein in the scalp follicle [4].
Within hair follicles, androgens act primarily on the dermal papilla cells, which contain AR with low capacity and high affinity. The molecular mechanism by which androgens exert their effect on the dermal papilla remains unclear. The current hypothesis is that the androgen–AR complex changes the production of autocrine and paracrine factors in the dermal papilla and thereby alters the growth of epithelial cells in the follicle [4]. A recent study found that androgens increase the synthesis and secretion of transforming growth factor beta (TGF-β) in the dermal papilla cells from the bald scalp and that TGF-β may be the androgen-induced growth inhibitor of co-cultured epithelial cells [35]. Wnt proteins are another possible regulatory factor that diffuses from the dermal papilla to the epithelium, triggering the androgenetic alopecia phenotype [36].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


