(1)
Department Obstetrics & Gynaecology, St George Hospital, Kogarah, New South Wales, Australia
Abstract
Before moving on to surgical treatment of stress incontinence, or management of prolapse, we must briefly consider the disorders of defecation.
Before moving on to surgical treatment of stress incontinence, or management of prolapse, we must briefly consider the disorders of defecation.
Many patients with urinary incontinence or prolapse have anal incontinence, recurrent straining with constipation, or other aspects of obstructive defecation. Because surgery may be considered for the defecation disorder, and in some pelvic floor units, these surgeries are performed simultaneously with bladder/prolapse procedures; such conditions are dealt with here.
Basic Physiology of Anal Continence and Defecation for the Gynecologist
Because the anal continence mechanism and the physiology of defecation are not part of normal registrar training in gynecology, the doctor who works in a urogynecology unit needs a basic outline before the treatment of defecation disorders can be understood.
Anal continence depends upon the following:
1.
Stool consistency (watery diarrhea alone can cause incontinence)
2.
The ability of the rectum to distend up to normal volumes
3.
The sensory input from the anal canal and rectum
4.
The strength and innervation of the internal and external anal sphincters
The first problem is unique to the bowel (although infected urine can cause bladder incontinence). The second and third problems are rather like those seen in an overactive bladder, where the bladder wall may be noncompliant and the subepithelial nerves are dysfunctional. The fourth problem is rather like that of stress incontinence (weak sphincters), except that the anal sphincters are more complex.
Several theories exist as to the mechanism of anal continence. In the 1970s, the main theory was that the normal puborectalis muscle caused an acute anorectal angle so that during rises in intra-abdominal pressure, the rectum was forced down upon the anal canal, with a “kink” at the puborectalis muscle so that feces were denied access to the anal canal. Arising from this concept, the operation of postanal repair was developed, to restore the anorectal angle and improve continence. This operation is still performed today, although success rates are very variable (see below).
Later studies showed that continence was really dependent upon the sphincters and the puborectalis muscle acting together. When the pressure in the rectum rises, the contractility of the anal sphincters increases, by neurological mechanisms. Thus, operations to repair the anal sphincter, which do not increase the anorectal angle, also improve continence.
Even later, it was realized that continence also depends on awareness of rectal filling. This gives one the ability to distinguish whether the rectal contents are gas (when they could be passed in a private location, not necessarily a toilet) or feces, in which case the external sphincter can be contracted voluntarily while looking for a toilet. The anal canal is highly able to discriminate light touch, pain, and temperature. In contrast, the rectum is not very sensitive to these impulses. Instead, rectal sensation is conveyed by stretch receptors within the pelvic floor muscles that respond to the bulk phenomenon of rectal distension.
Distension of the rectum (by feces or flatus) causes relaxation of the internal anal sphincter, along with contraction of the external anal sphincter. This allows the contents of the rectum to enter the sensitive anal canal (but prevents escape of the contents from the anus). Once the contents enter the anal canal, the nerves sense whether gas or feces are present. This is called the anal sampling reflex.
The sensitivity of the anal mucosa declines with age and menopause, partly explaining the increasing prevalence of anal incontinence in older women. Obviously, there is no surgical cure for denervation/declining sensitivity of the anal mucosa.
Filling of the rectum is normally first sensed at volumes of 10–70 ml. Maximum capacity is about 300 ml. Rectal distension initiates contractions of smooth muscle in the rectal wall, which cause the desire to defecate at “fullness.” This normal compliance of the rectal wall is reduced after pelvic irradiation, with inflammatory bowel disease, and sometimes after denervation following radical pelvic surgery.
Finally, strength and innervation of the sphincters are a vital component of continence. The internal anal sphincter (IAS) is continuous with the circular muscle in the wall of the rectum (see Fig. 8.1). It is in a constant state of tonic contraction, to promote continence. This provides the so-called high-pressure zone in the resting state, about 2 cm from the anal verge.
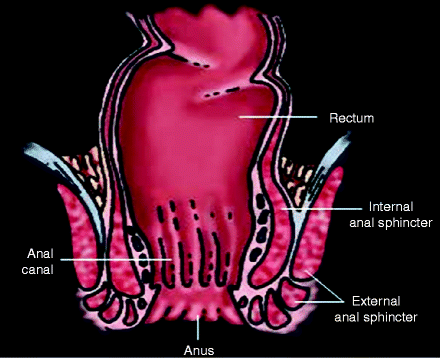

Figure 8.1
Anterior–posterior view of anorectal musculature
The high-pressure zone also receives a 15% contribution from the three “anal cushions” that have a rich arterial supply and behave like erectile tissue. They are engorged with blood when the IAS is relaxed and form a seal. Their pressure is higher in those with hemorrhoids and can be damaged by vigorous hemorrhoidectomy. Inadvertent division, or marked thinning, of the IAS (i.e., after some vaginal deliveries) is associated with fecal soiling in up to 40% of cases.
The external anal sphincter (EAS) is continuous with the puborectalis muscle (see Fig. 8.2). Although the EAS is striated muscle and is under voluntary control, it is also in a constant state of contraction to promote continence. During cough, the EAS tightens reflexively. Ultrasound studies of the EAS have shown that about 35% of women who delivered by forceps have a partial or complete laceration of the EAS, which generally does not recover and also contributes to anal incontinence.
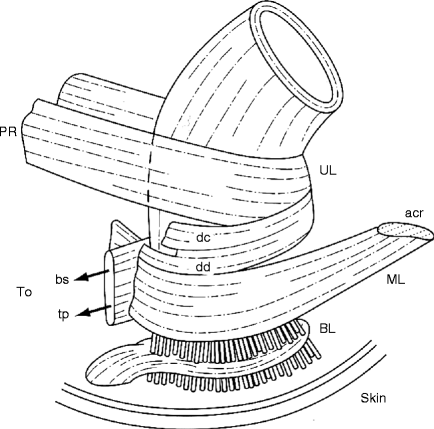

Figure 8.2
Lateral view of anorectal muscles. PR puborectalis, UL upper loop, DC decussating fibers of puborectalis that blend with the longitudinal muscle of the rectum, DD decussating fibers that join the perineal body, ML middle loop, ACR anococcygeal raphe, BS bulbospongiosus, TP deep transverse perinei, BL basal loop, perforated by fibers of the conjoint longitudinal layer (Reprinted with permission from Bogduk [2], Blackwell Publishing)
The motor innervation to the EAS is via the pudendal nerve. Prolonged bearing down in the second stage of labor is associated with a traction neurapraxia of the pudendal nerve, which may not recover. This partly explains the association between prolonged second stage and anal incontinence.
If rectal sensation is poor, and rectal compliance is reduced, and if the sphincters are also weak, then patients can experience anal incontinence before they even get the desire to defecate.
The Act of Defecation
The defecation mechanism is still not completely understood, despite extensive research:
Stool comes down from the sigmoid colon to the rectum, by peristalsis.
Stretch receptors in the pelvic floor detect the stool in the rectum, giving the urge to defecate.
The anal sampling reflex (internal sphincter relaxes, external sphincter stays closed) occurs; the anal mucosa senses whether stool or gas is present and conveys this to the brain.
If defecation is not socially convenient, the pelvic floor muscles and the puborectalis contract. This propels feces back up into the sigmoid colon, and the internal sphincter contracts again.
Once the toilet is reached, the pelvic floor muscles are relaxed while sitting on the toilet, allowing the perineum to descend.
Both sphincters are relaxed. Puborectalis opens.
The patient gives a Valsalva maneuver to raise intra-abdominal pressure.
The bolus of feces is expelled. Upon completion, a closing reflex tightens the external sphincter.
Overview of Anal Incontinence
Anal incontinence is really “the last taboo.” Patients are deeply ashamed if they soil themselves and usually consider it far worse than urinary incontinence. Questions must be phrased very tactfully; for example, do you ever lose bowel material on your underwear?
Anal incontinence is actually not uncommon. Large prevalence studies of community-dwelling women indicate that about 2–14% of women have anal incontinence, with up to 47% of those in nursing homes [13]. The most recent study indicates that 8.9% of noninstitutionalized women suffer from accidental loss of solid, liquid, or mucus incontinence in the month before questioning [13]. As can be seen from the discussion of the physiology of defecation, the pathophysiology of anal incontinence is often multifactorial. Full details about assessment of such patients are available in the text by Pemberton et al. [14]. The history assessment has been described in Chap. 1. The Wexner score should be used to measure severity of anal incontinence (shown in Chap. 5). Considerable detail about previous colorectal surgery, previous radiation, inflammatory bowel disease, etc., is needed. Physical examination requires attention to anal sphincter tone, perineal descent, pelvic innervation, etc.
We encourage any patient with regular fecal incontinence to be fully assessed by a dedicated colorectal surgeon. Such a surgeon is part of our unit so that case notes and nursing staff are shared. On the other hand, patients with minor incontinence to flatus or rare incontinence to liquid stool may benefit from conservative pelvic floor muscle training [5].
The common tests of anorectal function for patients with anal incontinence comprise the following:
Anorectal manometry tests the magnitude of the resting anal pressure at the high-pressure zone (85% comes from IAS rhythmic slow wave contractions, 15% from tonic contraction of EAS). The most common method is a water-perfused catheter containing four recording channels, to detect pressure at various points along the rectum/anal canal, with a balloon at the end. After testing baseline resting pressures, the patient is asked to cough (pressures should rise briefly, to prevent incontinence) and then to squeeze the EAS, which gives the voluntary “squeeze pressure.” The rectal balloon is then distended with fluid to elicit a brief drop in anal pressure, showing competency of the “sampling reflex.”
Pudendal nerve conduction studies test the innervation of the sphincters, by measuring whether the time taken to conduct a stimulus is delayed (the conduction latency). A stimulating electrode, mounted on a gloved finger, is inserted into the rectum; the fingertip is placed on the ischial spine (near the pudendal nerve), with a recording electrode at the external anal sphincter. The latency is the time taken for the electrical stimulus to reach the recording electrode. The test requires an experienced person to produce reliable measurements, and thus some units have discarded it, although it was a standard test for many years. Allen et al. [1]. used this test to provide the first evidence of intrapartum damage to the pudendal nerve, although long-term follow-up showed that most patients’ nerve conduction recovered over time. Prolonged straining at stool is also associated with prolonged pudendal nerve conduction times.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


