Physiology
Early in pregnancy, the amnionic cavity is filled with fluid that is similar in composition to extracellular fluid. During the first half of pregnancy, transfer of water and other small molecules takes place across the amnion—transmembranous flow, across the fetal vessels on placental surface—intramembranous flow, and across fetal skin. Fetal urine production begins between 8 and 11 weeks, but it does not become a major component of amnionic fluid until the second trimester. This latter observation explains why fetuses with lethal renal abnormalities may not manifest severe oligohydramnios until after 18 weeks. Water transport across the fetal skin continues until keratinization occurs at 22 to 25 weeks. This explains why extremely preterm infants can experience significant fluid loss across their skin.
With advancing gestation, four pathways play a major role in amnionic fluid volume regulation (Table 11-1). First, fetal urination is the primary amnionic fluid source by the second half of pregnancy. By term, fetal urine production may exceed 1 liter per day—such that the entire amnionic fluid volume is recirculated on a daily basis. Fetal urine osmolality is significantly hypotonic to that of maternal and fetal plasma and similar to that of amnionic fluid. Specifically, the osmolality of maternal and fetal plasma is approximately 280 mOsm/mL, whereas that of amnionic fluid is about 260 mOsm/L. This hypotonicity of fetal urine—and thus of amnionic fluid—accounts for significant intramembranous fluid transfer across and into fetal vessels on the placental surface, and thus into the fetus. This transfer reaches 400 mL per day and is a second regulator of fluid volume (Mann, 1996). In the setting of maternal dehydration, the resultant increase in maternal osmolality favors fluid transfer from the fetus to the mother, and then from the amnionic fluid compartment into the fetus (Moore, 2010).
TABLE 11-1. Amnionic Fluid Volume Regulation in Late Pregnancy
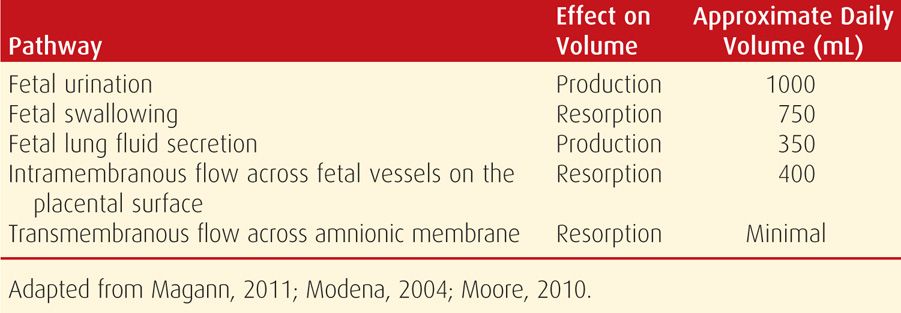
An important third source of amnionic fluid regulation is the respiratory tract. Approximately 350 mL of lung fluid is produced daily late in gestation, and half of this is immediately swallowed. Last, fetal swallowing is the primary mechanism for amnionic fluid resorption and averages 500 to 1000 mL per day (Mann, 1996). Impaired swallowing, secondary to either a central nervous system abnormality or gastrointestinal tract obstruction, can result in an impressive degree of hydramnios. The other pathways—transmembranous flow and flow across the fetal skin—account for a far smaller proportion of fluid transport in the second half of pregnancy.
 Measurement
Measurement
From a practical standpoint, the actual volume of amnionic fluid is rarely measured outside of the research setting. That said, direct measurement and dye-dilution methods of fluid quantification have contributed to an understanding of normal physiology. These measurements have further been used to validate sonographic fluid assessment techniques. The dye-dilution method involves injection of a small quantity of a dye such as aminohippurate into the amnionic cavity under sonographic guidance. The amnionic fluid is then sampled to determine the dye concentration and hence to calculate the fluid volume in which it was diluted.
Brace and Wolf (1989) reviewed 12 studies done through the 1960s in which amnionic fluid volume was assessed using these measurement techniques. Although fluid volume increased across gestation, they found that the mean value did not change significantly between 22 and 39 weeks—it was approximately 750 mL. There was considerable variation at each week of gestation, particularly in the mid-third trimester. At this time, the 5th percentile was 300 mL, and the 95th percentile approximated 2000 mL. In contrast, Magann and colleagues (1997) used dye-dilution measurements and found that the amnionic fluid volume continues to increase with advancing gestation. Specifically, the average fluid volume was approximately 400 mL between 22 and 30 weeks, doubling thereafter to a mean of 800 mL. The volume remained at this level until 40 weeks and then declined by approximately 8 percent per week thereafter. The two reports differed in the regression methodology employed, and despite different conclusions, both identified a wide normal range at each week of gestation, particularly in the third trimester. This normal variation has also been identified using semiquantitative sonographic methods, described next.
 Sonographic Assessment
Sonographic Assessment
Amnionic fluid volume evaluation is a component of every standard sonogram performed in the second or third trimester (Chap. 10, p. 199). Volume is typically assessed semiquantitatively, by measuring either a single pocket or the amnionic fluid index—AFI (Phelan, 1987). A qualitative or subjective estimate of amnionic fluid volume is also considered acceptable when performed by an experienced examiner (American College of Obstetricians and Gynecologists, 2011; American Institute of Ultrasound in Medicine, 2013). One limitation of subjective estimation, however, is that it does not permit a longitudinal assessment of trends in the amount or adequacy of fluid volume.
Single Deepest Pocket
This is also called the maximum vertical pocket. The ultrasound transducer is held perpendicular to the floor and parallel to the long axis of the pregnant woman. In the sagittal plane, the largest vertical pocket of fluid is identified. The fluid pocket may contain fetal parts or loops of umbilical cord, but these are not included in the measurement.
The normal range for single deepest pocket that is most commonly used is 2 to 8 cm, with values above and below this indicating hydramnios and oligohydramnios, respectively. These thresholds are based on data from Chamberlain and associates (1984) and correspond to the 3rd and 97th percentiles. This group also reported increased perinatal mortality rates among nonanomalous infants if the single deepest pocket was below the normal range. The fetal biophysical profile similarly uses a 2-cm single deepest vertical pocket threshold to indicate a normal amnionic fluid volume (American College of Obstetricians and Gynecologists, 2012). This is discussed in more detail in Chapter 17 (p. 341).
Other less commonly used thresholds to determine amnionic fluid volume adequacy involve measurement of a single pocket in both the vertical and transverse planes. Adequate fluid volume is defined as a 2 × 1 cm pocket, a 2 × 2 cm pocket, or a pocket that is at least 15 cm2 (Gramellini, 2004; Magann, 2000; Manning, 1990, 1993).
When evaluating twin pregnancies and other multifetal gestations, a single deepest pocket of amnionic fluid is assessed in each gestational sac, again using a normal range of 2 to 8 cm (Hernandez, 2012; Society for Maternal-Fetal Medicine, 2013).
Amnionic Fluid Index (AFI)
This was described by Phelan and coworkers (1987) more than 25 years ago, and it remains one of the most commonly used methods of amnionic fluid volume assessment. As with the single deepest fluid pocket measurement, the ultrasound transducer is held perpendicular to the floor and parallel to the long axis of the pregnant woman. The uterus is divided into four equal quadrants—the right- and left-upper and lower quadrants, respectively. The AFI is the sum of the single deepest pocket from each quadrant. A fluid pocket may contain fetal parts or umbilical cord loops, but these are not included in the measurement. Color Doppler is generally used to verify that no umbilical cord is included in the measurement. This may result in greater consistency and in reduction of intraobserver variation (Callen, 2008; Hill, 2003). It has been reported, however, that color Doppler use results in a lower AFI measurement, thus potentially leading to overdiagnosis of oligohydramnios (Magann, 2001).
The intraobserver variability of the AFI is approximately 1 cm and the interobserver variability about 2 cm. There are larger variations when fluid volumes are above the normal range (Moore, 1990; Rutherford, 1987b). A useful guideline is that the AFI is approximately three times the single deepest pocket of fluid encountered (Hill, 2003).
Normal AFI. Determination of whether the AFI is normal—that is, the thresholds for defining oligohydramnios and hydramnios—may be based on either a static numerical cut-off or a gestational age-specific percentile reference range.
The normal range for AFI that is most commonly used is 5 to 24 cm, with values above and below this indicating hydramnios and oligohydramnios, respectively. Rutherford and colleagues (1987a) reported an increased risk for adverse pregnancy outcomes with indices outside of this range. Moore and Cayle (1990) published normal curves for AFI values based on a cross-sectional evaluation of nearly 800 uncomplicated pregnancies. As shown in Figure 11-1, the mean AFI was found to be between 12 and 15 cm from 16 weeks until 40 weeks. Other investigators have published nomograms with similar mean values (Hinh, 2005; Machado, 2007).
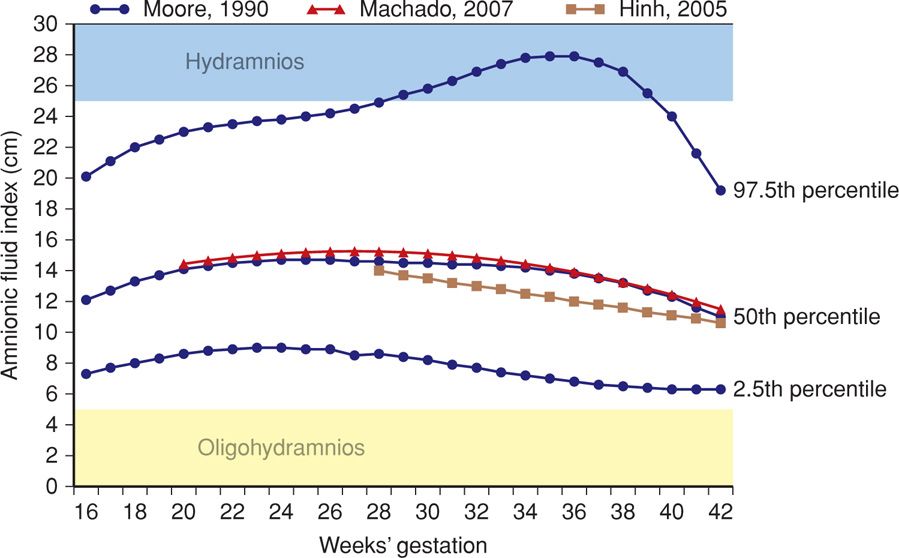
FIGURE 11-1 Amnionic fluid index (AFI) according to gestational-age-specific nomograms and threshold values. The blue curves represent the 2.5th, 50th, and 97.5th AFI percentile values, based on the nomogram by Moore (1990). Red and tan curves represent 50th percentile values for AFI from Machado (2007) and from Hinh and Ladinsky (2005), respectively. The light blue and yellow shaded bars indicate threshold values used to define hydramnios and oligohydramnios, respectively.
In the Moore (1990) nomogram, a threshold of 5 cm is < 2.5th percentile throughout the second and third trimesters. A value of 25 cm is > 95th percentile, but is not necessarily the 97.5th percentile, depending on gestational age. Using other published nomograms, a value of 25 cm exceeds the 97.5th percentile (Hinh, 2005; Machado, 2007). At this time, there is no consensus as to whether AFI nomogram use improves prediction of adverse outcome compared with use of a numerical threshold alone, and both are considered acceptable.
HYDRAMNIOS
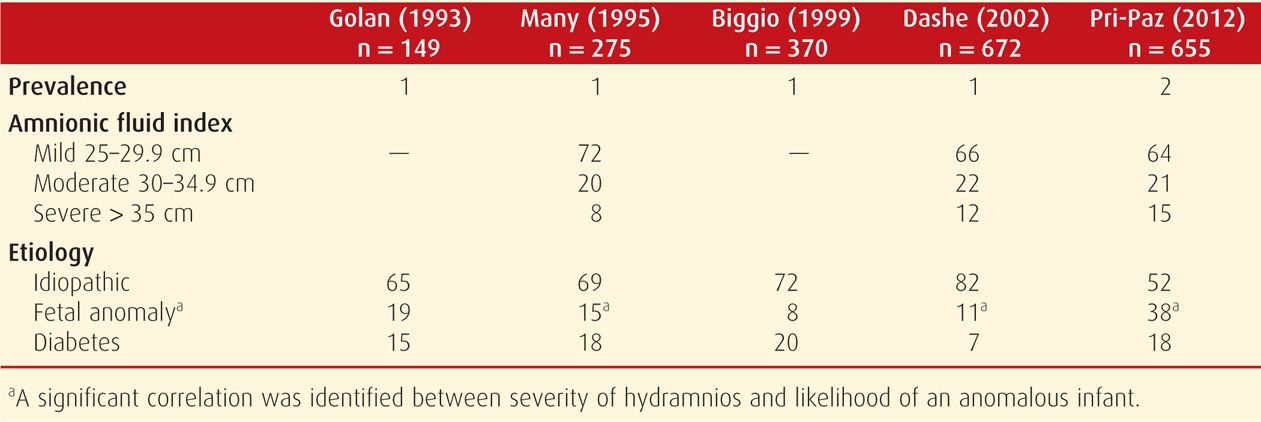
This is an abnormally increased amnionic fluid volume, and it complicates 1 to 2 percent of pregnancies (Biggio, 1999; Dashe, 2000; Magann, 2007; Pri-Paz, 2012). Also termed polyhydramnios, hydramnios may be suspected if the uterine size exceeds that expected for gestational age. The uterus may feel tense, and palpating fetal small parts or auscultating fetal heart tones may be difficult. An extreme example is shown in Figure 11-2.
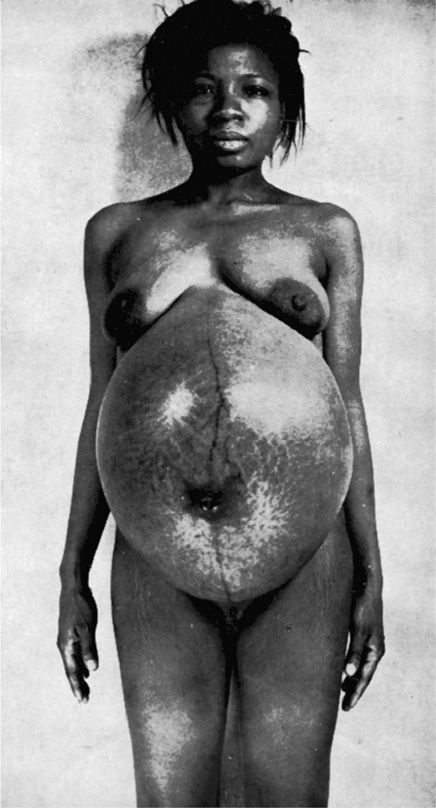
FIGURE 11-2 Severe hydramnios—5500 mL of amnionic fluid was measured at delivery.
Hydramnios may be further categorized according to degree. Such categorization has been primarily used in research studies to stratify risks. Several groups have termed hydramnios as mild if the AFI is 25 to 29.9 cm, moderate if 30 to 34.9 cm, and severe if 35 cm or more (Dashe, 2002; Lazebnik, 1999; Pri-Paz, 2012). Mild hydramnios is the most common, comprising approximately two thirds of cases. Moderate hydramnios accounts for about 20 percent, and severe hydramnios approximately 15 percent. Using the single deepest pocket of amnionic fluid, criteria for mild is 8 to 9.9 cm, moderate is 10 to 11.9 cm, and severe hydramnios, ≥ 12 cm. An example of severe hydramnios seen sonographically is shown in Figure 11-3. These definitions fit the general rule that the AFI is approximately three times the measurement of the single deepest fluid pocket (Hill, 2003). In general, severe hydramnios is far more likely to have an underlying etiology and to have consequences for the pregnancy than mild hydramnios—which is frequently idiopathic and benign.
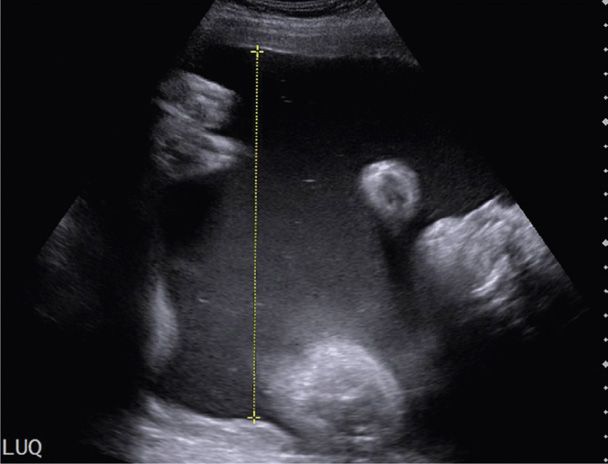
FIGURE 11-3 Sonogram of severe hydramnios at 35 weeks in a pregnancy complicated by fetal aqueductal stenosis. This pocket of amnionic fluid measures more than 15 cm, and the amnionic fluid index measured nearly 50 cm.
 Etiology
Etiology
Common underlying causes of hydramnios include fetal congenital anomalies in approximately 15 percent and diabetes in 15 to 20 percent (Table 11-2). Congenital infection and red blood cell alloimmunization are less frequent reasons. Infections that may present with hydramnios include cytomegalovirus, toxoplasmosis, syphilis, and parvovirus (Chaps. 64 and 65). Hydramnios is often a component of hydrops fetalis, and several of the above etiologies—selected anomalies, infections, and alloimmunization—may result in a hydropic fetus and placenta. The underlying pathophysiology in such cases is complex but is frequently related to a high cardiac-output state. Severe fetal anemia is the classic example. Because the etiologies of hydramnios are so varied, hydramnios treatment also varies and is tailored in most cases to the underlying cause. These etiologies are discussed further in Chapter 15 (p. 315) and in other chapters covering specific topics.
TABLE 11-2. Hydramnios: Prevalence and Associated Etiologies—Values in Percent
Diabetes Mellitus
The amnionic fluid glucose concentration is higher in diabetic women than in those without diabetes, and the amnionic fluid index may correlate with the amnionic fluid glucose concentration (Dashe, 2000; Spellacy, 1973; Weiss, 1985). Such findings support the hypothesis that maternal hyperglycemia causes fetal hyperglycemia, with resulting fetal osmotic diuresis into the amnionic fluid compartment.
Congenital Anomalies
Various anomalies may be found in the setting of hydramnios, and some are more characteristically linked with it than others. Because of this association, identification of hydramnios is an indication for targeted sonography. Many of the anomalies described next are displayed sonographically in Chapter 10 (p. 200).
Severe central nervous system abnormalities, such as anencephaly, hydranencephaly, or holoprosencephaly, can result in hydramnios due to impaired fetal swallowing. Fetal neuromuscular disorders such as myotonic dystrophy also may lead to excessive amnionic fluid. Obstruction of the fetal upper gastrointestinal tract—esophageal or duodenal atresia—is often associated with hydramnios. Other obstructive causes include clefts, micrognathia, congenital high-airway obstruction sequence, and fetal neck masses. Severe fetal thoracic abnormalities, such as diaphragmatic hernia, cystic adenomatoid malformation, and pulmonary sequestration, may be associated with hydramnios due to mediastinal shift and impaired swallowing, occasionally with development of hydrops. A common fetal renal anomaly, ureteropelvic junction obstruction, may at times result in paradoxical hydramnios. And although rare, tumors such as fetal sacrococcygeal teratoma, fetal mesoblastic nephroma, and large placental chorioangiomas are frequently accompanied by abnormally increased amnionic fluid volume.
The degree of hydramnios is associated with the likelihood of an anomalous infant (Many, 1995; Pri-Paz, 2012). For example, at Parkland Hospital, the prevalence of an anomalous infant was approximately 8 percent with mild hydramnios, 12 percent with moderate hydramnios, and more than 30 percent with severe hydramnios (Dashe, 2002). If no abnormality was detected with targeted sonography, the likelihood of a major anomaly identified at birth was 1 to 2 percent if the hydramnios was mild or moderate but exceeded 10 percent if hydramnios was severe. Dorleijn and associates (2009) have also reported an increased risk for abnormalities detected in the first year of life with apparent idiopathic hydramnios. The anomaly risk is particularly high with hydramnios coexistent with fetal-growth restriction (Lazebnik, 1999). If a fetal abnormality is encountered concurrent with hydramnios, amniocentesis should be considered, because the aneuploidy risk is significantly increased (Dashe, 2002; Pri-Paz, 2012).
Although amnionic fluid volume abnormalities are associated with fetal malformations, the converse is not usually the case. In the Spanish Collaborative Study of Congenital Malformations that included more than 27,000 anomalous infants, only 4 percent were complicated by hydramnios, and another 3 percent with oligohydramnios (Martinez-Frias, 1999).
Multifetal Gestation
Hydramnios is generally defined in multifetal gestations as a single deepest amnionic fluid pocket measuring 8 cm or more. It may be further characterized as moderate if the single deepest pocket is at least 10 cm and severe if this pocket is at least 12 cm. In monochorionic pregnancies, hydramnios of one sac and oligohydramnios of the other are diagnostic criteria for twin-twin transfusion syndrome, which is discussed in Chapter 45 (p. 904). In a review of nearly 2000 twin gestations, Hernandez and coworkers (2012) identified hydramnios in 18 percent of both monochorionic and dichorionic pregnancies. As in singletons, severe hydramnios was more strongly associated with fetal abnormalities. In the absence of an abnormality, pregnancy risks were not generally increased compared with twins with normal amnionic fluid volume.
Idiopathic Hydramnios
When there is no obvious cause of hydramnios it is considered idiopathic. As shown in Table 11-2, this accounts for up to 70 percent of cases (Golan, 1993; Many, 1995; Panting-Kemp, 1999). Pregnancies with idiopathic hydramnios have been reported to have at least twice the likelihood of infant birthweight exceeding 4000 g (Lazebnik, 1999; Magann, 2010; Maymon, 1998). A rationale for this association is that larger infants have higher urine output, by virtue of their increased volume of distribution, and fetal urine is the largest contributor to amnionic fluid volume. Mild, idiopathic hydramnios is most commonly a benign finding, and associated pregnancy outcomes are usually good.
 Complications
Complications
Unless hydramnios is severe or develops rapidly, maternal symptoms are infrequent. With chronic hydramnios, fluid accumulates gradually, and a woman may tolerate excessive abdominal distention with relatively little discomfort. Acute hydramnios, however, tends to develop earlier in pregnancy. It may result in preterm labor before 28 weeks or in symptoms that become so debilitating as to necessitate intervention.
Symptoms may arise from pressure exerted within the overdistended uterus and upon adjacent organs. When distention is excessive, the mother may suffer dyspnea and orthopnea to such a degree that she may be able to breathe comfortably only when upright (see Fig. 11-2). Edema may develop as a consequence of major venous system compression by the enlarged uterus, and it tends to be most pronounced in the lower extremities, vulva, and abdominal wall. Rarely, oliguria may result from ureteral obstruction by the enlarged uterus (Chap. 53, p. 1064). Maternal complications such as these are typically associated with severe hydramnios from an underlying etiology.
Maternal complications associated with hydramnios include placental abruption, uterine dysfunction, and postpartum hemorrhage. Placental abruption is fortunately infrequent. It may result from the rapid decompression of an overdistended uterus that follows fetal-membrane rupture or therapeutic amnioreduction. With prematurely ruptured membranes, a placental abruption occasionally occurs days or weeks after amniorrhexis. Uterine dysfunction consequent to overdistention may lead to postpartum atony and, in turn, postpartum hemorrhage.
 Pregnancy Outcomes
Pregnancy Outcomes
Some outcomes that have been reported to be increased with hydramnios include cesarean delivery rate, birthweight > 4000 g, and importantly, perinatal mortality rate. The cesarean delivery rate is increased approximately threefold when hydramnios has been identified, and the perinatal mortality rate rises approximately fourfold (Biggio, 1999; Hill, 1987; Maymon, 1998). Pri-Paz and colleagues (2012) found that pregnancies with severe hydramnios were at particular risk, but reported no perinatal deaths with idiopathic hydramnios.
Risks appear to be compounded when a growth-restricted fetus is identified with hydramnios. Erez and associates (2005) reported that this combination was independently associated with a 20-fold increase in the perinatal mortality rate. The combination also has a recognized association with trisomy 18 (Sickler, 1997).
Considering that uterine distention from hydramnios may result in uterine size approaching that of a term gestation, preterm delivery is a logical concern. Somewhat surprisingly, studies of idiopathic hydramnios have generally found no association with preterm birth (Magann, 2010; Many, 1995; Panting-Kemp, 1999). Conversely, severe hydramnios and hydramnios concurrent with recognized fetal abnormalities have been linked with preterm birth (Many, 1995; Pri-Paz, 2012).
 Management
Management
As noted previously, hydramnios etiologies are varied, and treatment is directed in most situations to the underlying cause. Occasionally, severe hydramnios may result in early preterm labor or the development of maternal respiratory compromise. In such cases, large-volume amniocentesis—termed amnioreduction—may be needed. The needle insertion technique is the same as for amniocentesis, described in Chapter 14 (p. 297). However, either an evacuated container bottle or a larger syringe is connected to the needle via sterile intravenous tubing with a stopcock. In general, approximately 1000 to 1500 mL of fluid is slowly withdrawn during approximately 30 minutes, depending on the severity of hydramnios and gestational age. The goal is to restore amnionic fluid volume to upper normal range (p. 232). Hydramnios severe enough to necessitate amnioreduction almost invariably has an underlying etiology, and subsequent amnioreduction procedures may be required as often as weekly or even semiweekly. Importantly, amnioreduction is typically performed later in gestation and carries additional risks of membrane rupture, preterm labor or its exacerbation, and placental abruption.
OLIGOHYDRAMNIOS
This is an abnormally decreased amount of amnionic fluid. Oligohydramnios complicates approximately 1 to 2 percent of pregnancies (Casey, 2000; Petrozella, 2011). Unlike hydramnios, which is often mild and confers a benign prognosis in the absence of an underlying etiology, oligohydramnios is a cause for concern. When no measurable pocket of amnionic fluid is identified, the term anhydramnios may be used.
The sonographic diagnosis of oligohydramnios is usually based on an AFI ≤ 5 cm or on a single deepest pocket of amnionic fluid ≤ 2 cm (American College of Obstetricians and Gynecologists, 2012). The diagnosis also may be based on an AFI below the 5th or 2.5th percentile determined by a gestational-age-specific nomogram. Or, it may be based on subjective assessment of decreased amnionic fluid volume. In the Moore nomogram, a threshold of 5 cm is below the 2.5th percentile throughout the second and third trimesters (see Fig. 11-1). When evaluating twin pregnancies for twin-twin transfusion syndrome, a single deepest pocket ≤ 2 cm is used to define oligohydramnios (Society for Maternal-Fetal Medicine, 2013).
In general, no one criterion is considered superior to the others. As discussed subsequently, however, use of AFI rather than single deepest pocket will identify more pregnancies as having oligohydramnios, albeit without evidence of pregnancy outcome improvement (Nabhan, 2010).
 Etiology
Etiology
Pregnancies complicated by oligohydramnios include those in which the amnionic fluid volume has been severely decreased since the early second trimester and those in which the fluid volume was normal until near-term or even full-term. The prognosis depends heavily on the underlying etiology and is variable. Whenever oligohydramnios is diagnosed, it becomes an important consideration in management decisions.
Early-Onset Oligohydramnios
When amnionic fluid volume is abnormally decreased from the early second trimester, it may reflect a fetal abnormality that precludes normal urination, or it may represent a placental abnormality severe enough to impair perfusion. In either circumstance, the prognosis is poor. Second-trimester rupture of the fetal membranes may result in oligohydramnios—and should be excluded. More commonly, membrane rupture presents with fluid leakage, vaginal bleeding, or uterine contractions. With early-onset oligohydramnios, targeted sonography should be offered to assess for fetal abnormalities.
Oligohydramnios after Midpregnancy
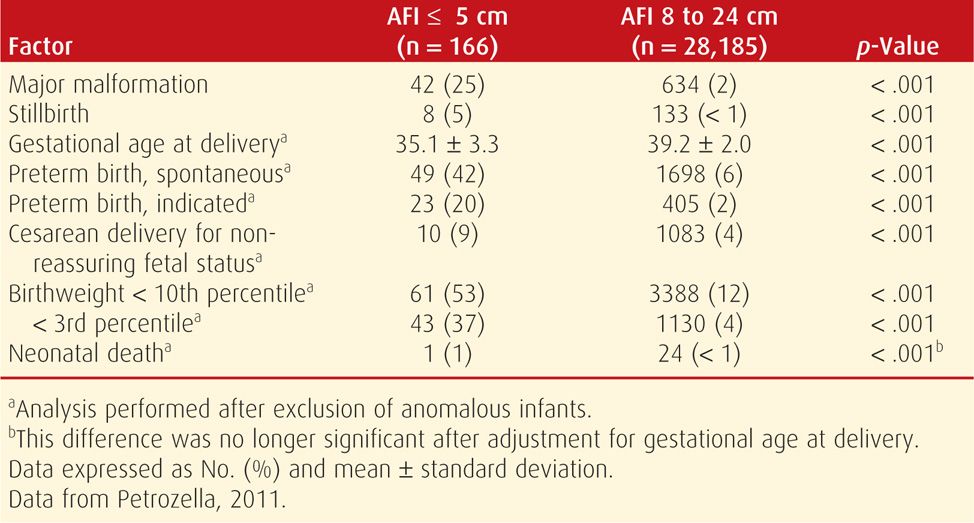
When amnionic fluid volume becomes abnormally decreased in the late second or in the third trimester, it more likely is associated with fetal-growth restriction, a placental abnormality, or a maternal complication such as preeclampsia or vascular disease (Table 11-3). In such cases, the underlying etiology is often presumed to be uteroplacental insufficiency, which can impair fetal growth and reduce fetal urine output. Exposure to selected medications has also been linked with oligohydramnios as discussed subsequently. Investigation of third-trimester oligohydramnios generally includes evaluation for membrane rupture and sonography to assess growth. Umbilical artery Doppler studies may be performed if growth restriction is identified (Chap. 10, p. 219).
TABLE 11-3. Pregnancy Outcomes in Women Diagnosed with Oligohydramnios between 24 and 34 Weeks
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


