CHAPTER 16 Amenorrhoea, oligomenorrhoea and hypothalamic–pituitary dysfunction
Introduction
Amenorrhoea and oligomenorrhoea are symptoms of ovarian and reproductive dysfunction. Patients thus commonly present to the gynaecologist with complaints of problematic menstruation or fertility delay. This chapter provides an overview of the current understanding and general management of the associated disorders of the hypothalamic–pituitary–ovarian (HPO) axis that result in amenorrhoea and oligomenorrhoea. These symptoms are also features of the polycystic ovary syndrome (PCOS), and a detailed description of the symptoms, diagnosis and management of PCOS is provided in Chapter 18.
Definitions
Normal menstruation
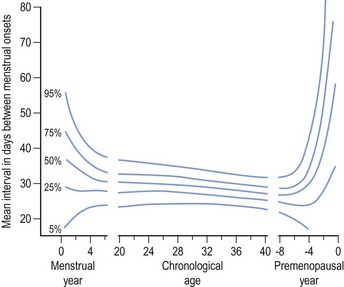
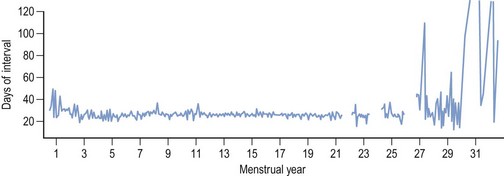
Regular monthly menstruation is a phenomenon of modern society. Furthermore, the availability of effective contraception has enabled couples to choose both the size and timing of their desired family. The classic data (derived from 22,754 calendar years of experience) from the studies of Treloar et al (1967) demonstrated that each woman has her own central trend and variation in menstrual cycle length, both of which change with age (Figure 16.1). The first (and last) few years of menstrual life are marked by a variable pattern of mixed short and long intervals, with a characteristic transition into and out of the more regular pattern of middle life (Figure 16.2). The length, regularity and frequency of normal menstrual cycles have been described in both population and observational studies (Harlow and Ephross 1995, Fraser and Inceboz 2000). Mean menstrual cycle length between the ages of 20 and 34 years varies between 28 and 30.7 days (range 19.7–43.5 between the 5th and 95th centiles). Physiologically regular menses indicate cyclical ovarian activity, in turn dependent upon an intact HPO axis. Aberrations in menstrual pattern are thus an indication of disorder of ovarian function. The average age of the menarche is 12.8 years, but this has been gradually decreasing. Factors such as ethnic origin, socio-economic status and nutrition can affect age of menarche. With obesity presently such a prominent problem, it is interesting to note that there is a relationship between age of menarche and body mass index (BMI), with early menarche associated with raised BMI (Golden and Carlson 2008).
Causes of Amenorrhoea
Causes of amenorrhoea are classified according to a systematic endocrine approach and are listed in Box 16.1 (Baird 1997). Disorders in other endocrine systems, such as thyroid disease and adrenal disease, may result in amenorrhoea.
Box 16.1
Classification of causes of amenorrhoea
Adapted from Baird, Amenorrhoea. Lancet 350:275–279. © The Lancet Ltd 1997.
Physiological amenorrhoea
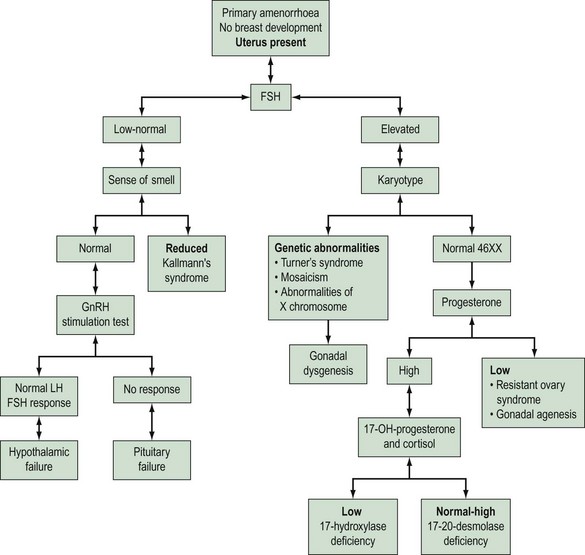
Amenorrhoea is physiological at certain critical times in a woman’s life, these being prepuberty, during pregnancy and lactation, and in the postmenopausal period. Amenorrhoea, if not physiological, has an estimated prevalence in the female population of reproductive age of 1.8–3% (Pettersson et al 1973). Amenorrhoea may be either primary or secondary. The prevalence of secondary amenorrhoea is in the order of 2–5% (Singh 1981). The aetiology of primary and secondary amenorrhoea may be similar. Causes of primary amenorrhoea are listed in Box 16.2. In practice, investigation of primary amenorrhoea is usually initiated by the age of 14 years if there is evidence of delayed puberty (absent secondary sexual characteristics and absent menses), or no menstruation within 4 years of the onset of adrenarche and thelarche (Zreik and Olive 1998). The diagnosis of cause of primary amenorrhoea may be categorized depending on whether or not the uterus is present, and whether or not there is breast development. Zreik and Olive (1998) described a very useful scheme to aid diagnosis of primary amenorrhoea (Figure 16.3).
Anatomical causes
Anatomical abnormalities of the genital tract account for approximately 1% of cases of amenorrhoea. The anatomical causes of amenorrhoea are summarized in Box 16.3. In girls with breast development but evidence of an absent uterus, two disorders need to be considered. First, congenital absence of the uterus (Müllerian agenesis, Mayer–Rokitansky–Kuster–Hauser syndrome) is due to an early development failure of the Müllerian system. Affected girls have a normal XX karyotype, normal ovaries and secondary sexual characteristics. The vagina is absent or hypoplastic. Magnetic resonance imaging is a useful adjunct diagnostic test for establishment of the diagnosis, thereby avoiding laparoscopy. Since anomalies of the Wolffian duct system may be present in these patients, an intravenous urogram is an important investigation for this condition. The second disorder to consider is testicular feminization or androgen insensitivity, an X-linked inherited disorder. Patients have a 46XY karyotype, a female phenotype and undescended testes. The uterus is absent and there is a short, blind vaginal pouch. The X-linked androgen receptor is essential for androgen action, leading to normal primary male sexual development prior to birth (masculinization). Thus, androgen receptor dysfunction in XY individuals results in androgen insensitivity syndrome. Diagnosis of this syndrome is established on clinical findings, endocrine investigations and, if possible, family history. There are three phenotypes: complete androgen insensitivity syndrome (CAIS), partial androgen insensitivity syndrome (PAIS) and mixed androgen insensitivity syndrome (MAIS). CAIS is most often diagnosed on clinical findings and laboratory investigations, and PAIS and MAIS usually require a family history consistent with X-linked inheritance (Gottlieb et al 1999). Androgen insensitivity may be caused by several mutations of the androgen receptor (Gottlieb et al 1999), resulting in a lack of androgenization during sexual differentiation (Imperato-McGinley 1995). Development of the uterus and upper vagina is inhibited, as the secretion of anti-Müllerian hormone (AMH) by the testes is normal in these patients. Recently, due to nationwide cooperation between paediatric endocrinologists in the UK, an extensive database (278 cases) of phenotypic features, androgen receptor binding, and mutational analysis of cases of intersex and ambiguous genitalia has been established (Ahmed et al 2000). All cases of PAIS presented within the first month of life. The median age for presentation of individuals with CAIS was 1 year. The gonads were removed before puberty in 66% of cases with CAIS, and after puberty in 29% of cases. The indication for gonadal removal is the high incidence of neoplasia (5%).
Box 16.3 Anatomical causes of amenorrhoea
A further anatomical cause of primary amenorrhoea is the presence of an imperforate hymen that obstructs the outflow of menses. Girls commonly present with cyclical pelvic pain and a delayed menarche. Secondary amenorrhoea can result from anatomical abnormalities; the endometrium may be destroyed as a result of infection (e.g. pelvic tuberculosis) or iatrogenically, as a result of an endometrial ablation procedure. Complications of uterine curettage can also result in amenorrhoea. An example of this is Asherman’s syndrome (Asherman 1948), which is defined by the presence of intrauterine permanent adhesions, obliterating the uterine cavity either partially or completely. The main cause of this syndrome is the procedure of endometrial curettage required to treat secondary postpartum haemorrhage due to retained placental products. It has also been suggested that missed miscarriages are an important risk factor for the development of adhesions.
Hypothalamic–pituitary dysfunction (endocrine causes)
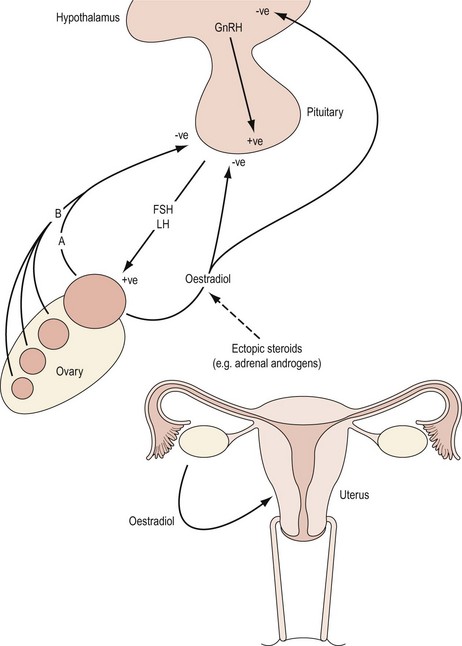
The hypothalamic–pituitary axis is regulated at two levels. At a higher level, gonadotrophin-releasing hormone (GnRH) neurones within the hypothalamus are stimulated by afferent inputs from the central nervous system. Furthermore, there is endocrine control of GnRH synthesis and secretion by means of gonadal feedback mechanisms and GnRH itself. At a lower level, the output of FSH and luteinizing hormone (LH) by the gonadotropes in the anterior pituitary reflects GnRH activity. Gonadotrophin synthesis and secretion are, in turn, influenced by endocrine feedback from the ovaries as well as paracrine mechanisms and other extrinsic factors. Endocrine causes of oligomenorrhoea and amenorrhoea include disorders of the HPO axis that are summarized in Box 16.4. Disturbances in menstrual pattern will be the consequence of either a structural or functional defect in the tightly controlled feedback system involving the hypothalamus, anterior pituitary and ovary (Baird 1997; Figure 16.4). If the uterus is present and there is no breast development, absence of menstruation may be due to failure of secretion of GnRH or to failure of pituitary or gonadal function (see Figures 16.3 and 16.4). Hypothalamic amenorrhoea suggests an intact HPO axis.
Kallman’s syndrome
Kallman’s syndrome, occurring in 1 : 50,000 girls (Kallman et al 1944), has the associated symptom of anosmia and is an inherited autosomal-dominant or X-linked autosomal-recessive anomaly. The impairment of olfactory sensation is often subtle. Women with this condition typically present with primary amenorrhoea and poor development of secondary sexual characteristics. If untreated, infertility results. The gonadotrophin deficiency is due to an inability to activate pulsatile GnRH secretion (Leiblich et al 1982). Five Kallman’s syndrome genes have been identified to date. The syndrome appears to result from insufficient cell signalling through fibroblast growth factor receptor 1 (FGFR1), in which integrins play a role in signalling, and the prokineticin receptor 2, a G-protein coupled receptor essential for normal development of the olfactory bulbs and sexual maturation (Abreu et al 2008). Some cases are due to a mutation in the KAL gene situated on the p22.3 region of the X chromosome (tip of the short arm) that codes for an adhesion-molecule-like X chromosome (Legouis et al 1991). This protein has homology with fibronectin and plays a role in the migration of GnRH-like neurones from the nasal pit to the hypothalamus (Rugarli and Ballabio 1993). KAL1 gene mutations account for 33–70% of cases of the X-linked form of Kallman’s syndrome. Ovulation induction can achieve pregnancy for these women, but requires both exogenous FSH and LH to successfully stimulate follicular maturation and ovarian steroidogenesis.
Hypogonadotrophic hypogonadism
Female hypogonadism refers to deficient or abnormal function of the HPO axis that clinically presents with menstrual cycle disturbances. Female hypogonadism can be due to a congenital or acquired cause, and the defect can be at the level of the hypothalamus, pituitary or ovary. It is characterized by reduced secretion of FSH (although this can be normal) and LH. There is a consequent failure of follicular development and oestradiol production by the ovaries. A hypo-oestrogenic state thus prevails. If the situation is present before puberty, the girl will present with primary amenorrhoea and a lack of secondary sexual features. Usually, no organic lesion is identified in the hypothalamus or anterior pituitary, and the situation is considered to be idiopathic. Mutations in three genes account for 15–20% of all cases of idiopathic hypogonadotrophic hypogonadism: KAL1, GNRHR and FGFR1. Nearly all mutations are point mutations (Pederson-White et al 2008). Severe weight loss, psychological stress and chronic debilitating disease are all associated with a cessation of hypothalamic function, and are conditions that will be addressed later.
Hypothalamic and pituitary lesions
The most likely hypothalamic lesion to present to a gynaecologist is a craniopharyngioma. Compression of the hypothalamus will suppress GnRH secretion and interrupt portal flow of GnRH in the pituitary stalk. The peripubertal period is the most common age for presentation. The lesions are cystic and often calcified, and are readily recognizable on a lateral skull radiograph or with imaging techniques, such as magnetic resonance imaging. Other tumours which may affect hypothalamic–pituitary function are gliomas (which may arise from the optic tract), meningiomas, endodermal sinus tumour (yolk sac carcinoma), which secretes α-fetoprotein, and congenital hamartomas composed of GnRH neurosecretory cells which can lead to precocious puberty (Yen 1999). Congenital absence of the anterior pituitary gland along with other midline structural defects is extremely rare. Primary deficiency of pituitary hormone secretion is also very uncommon. Growth hormone deficiency may occur in isolation or with panhypopituitary dwarfism. Pituitary failure may be secondary to other organic disease, such as pituitary adenoma, mumps, encephalitis, infarction (Sheehan’s syndrome, secondary to major obstetric haemorrhage) and irradiation. Pituitary cells are relatively resistant to irradiation compared with other brain tissues that are more radiosensitive. Disturbance of pituitary function may therefore be indirectly due to hypothalamic damage. Subjects with gonadal failure are oestrogen deficient and have elevated gonadotrophins (hypergonadotrophic hypogonadism). Causes of gonadal failure are listed in Box 16.5 and will be addressed in detail later. One of the most common chromosomal causes of primary amenorrhoea is Turner’s syndrome. Enzyme-deficiency states associated with primary amenorrhoea include galactosaemia and 17-hydroxylase deficiency.
Central nervous system–hypothalamic disturbance
The modulating influence of extrahypothalamic brain centres on the pulsatile nature of hypothalamic GnRH secretion is addressed in Chapter 15. Psychological disorders account for approximately one-third of cases of amenorrhoea due to central nervous system–hypothalamic disturbance. Functional disorders of the hypothalamic–pituitary axis that cause amenorrhoea result from weight loss, extreme exercise and psychological stress. In each of these situations, there is a decrease in GnRH neuronal activity in the hypothalamus with a subsequent decrease in gonadotrophin secretion (FSH and LH). Ovarian follicular development and ovulation fail to occur if LH pulsatility is less frequent than every 2 h (Baird 1997).
Weight-related amenorrhoea
Marked weight loss, such as that occurring with anorexia nervosa, may result in amenorrhoea. However, menstrual irregularity is an associated feature of all eating disorders rather than being restricted to anorexia nervosa alone. The amount of weight loss that may result in cessation of menstruation varies from a few kilograms in an adolescent who is dieting to a loss of up to 50% of body weight in women with anorexia nervosa (Warren 1995). Regular menses are unlikely to occur in subjects with BMI <19 kg/m2 (Balen et al 1995). It has been reported that nearly one-fifth of the body mass should be adipose tissue for ovulatory cycles to be sustained (Frisch 1984). The rate of loss of weight seems to be important, and rapid loss is frequently associated with psychological disturbance. The chronic hypo-oestrogenic state that becomes established in long-standing amenorrhoea carries significant risk of premature osteoporosis and cardiovascular disease. Irregular cycles and anovulation are also common in PCOS and may be due to the commonly associated raised BMI. The insulin resistance with PCOS which is exacerbated by an increase in BMI affects the intraovarian response to gonadotrophins (Greer et al 1980).
Exercise-related amenorrhoea
Irregularities of menstrual pattern are reported in association with many competitive sporting activities. There is typically a progressive failure of regular menses with anovulatory cycles and amenorrhoea and, in prepubertal girls, a delayed menarche. Usually, the degree of menstrual aberration reflects the intensity and length of sporting activity (Yen 1999). Secondary amenorrhoea and oligomenorrhoea are also common in professional dancers (Warren et al 1986). Women with hypothalamic amenorrhoea associated with an excessive exercise habit, weight loss and stress have hypercortisolism, on account of raised corticotrophin-releasing hormone (CRH) and adrenocorticotrophic hormone. Consequently, there is likely to be disruption of reproductive function as CRH directly inhibits GnRH secretion, possibly via increased endogenous opioid secretion (Speroff et al 1999a). A detailed study was reported by Laughlin and Yen (1996) which addressed the interactions between energy balance and regulators of metabolic fuel and the association with reduced LH pulsatility in women with exercise-induced menstrual dysfunction. Notable differences were observed in nutritional intake, insulin/glucose dynamics, the somatotrophic axis and LH pulsatility with both degree of exercise and menstrual pattern. Those athletes who were amenorrhoeic demonstrated an increase in insulin sensitivity and a reduced hypoglycaemic effect of insulin-like growth factor 1 (IGF-1), along with raised growth hormone and cortisol concentrations. The authors suggest that there is a cascade of glucoregulatory adaptations designed to redistribute metabolic fuel and thereby conserve protein. Furthermore, the reduced GnRH/LH pulse generator activity is in response to both a reduced stimulatory effect of IGF-1 (due to increased IGF-binding protein 1) and central negative effects of corticotrophin-releasing factor. The outlook for women with stress-/exercise-related amenorrhoea is very good if recognized early. Most women see a return of ovulatory cycles with weight gain or reduction in levels of stress or exercise habit (Speroff et al 1999a). Hormone replacement may be indicated for women with long-standing hypothalamic amenorrhoea as there will be a risk of bone loss and cardiovascular changes.
Metabolic hormones regulating reproductive function
Kisspeptins, and their cognate receptor gpr-54, were first found to regulate the hypothalamic–pituitary–gonadal axis in 2003, when two groups demonstrated that mutations in gpr-54 cause idiopathic hypogonadotropic hypogonadism characterized by delayed or absent puberty (Roseweir and Millar 2009). KiSS1 neurones have recently been suggested to mediate leptin’s effect on the reproductive system by encoding hypothalamic neuropeptides. Leptin is the primary product of the ob gene and is a 167 amino acid peptide made exclusively in adipose tissue; it acts on the hypothalamus. It is likely that leptin plays a central role in energy production, reproduction and weight, and it has been proposed as a mediator between adipose tissue and the gonads (Matkovic et al 1997). In both eating disorders and excessive exercise, amenorrhoea results from an adaptive response to an energy deficit, partially mediated by leptin. A critical blood leptin level has been reported as necessary to trigger reproductive function in women, suggesting a threshold effect. In this context, severe weight loss is known to result in subnormal gonadotrophin concentrations. Leptin receptors have been identified in the hypothalamus, and leptin inhibits neuropeptide Y (NPY) synthesis and release. A link between leptin and GnRH neurones is, in part, mediated by NPY. Secondary leptin deficiency can present as weight-related amenorrhoea in women (Conway and Jacobs 1997). Speroff et al (1999a) noted that CRH is elevated in stress- and, particularly, weight-loss-related amenorrhoea. It has been proposed that the decrease in leptin and increase in NPY described in stress-related weight loss are inadequate to suppress the stress-induced increase in CRH. Moreover, the increase in CRH and resulting hypercortisolism exacerbate the increase in metabolism and weight loss. Research leading to a better understanding of the metabolic regulation of reproductive function has implications for the prevention and management of reproductive dysfunction and its associated comorbidities.
Neurological and psychiatric disorders
Epilepsy, bipolar disorder and migraines are common disorders which can be associated with disturbances in menstrual function in adolescent girls. With epilepsy, it is thought that both the disease itself and the treatment medications contribute to the aetiology of the menstrual abnormality by disturbing the HPO axis. Many antipsychotic drugs are also dopamine antagonists, and can cause prolactin levels to increase up to 10-fold, again resulting in inhibition of the HPO axis (see Box 16.6).