Ludwig’s classification of FPHL. Adapted with permission from Ludwig [30], John Wiley and Sons ©.
Evaluation
Diagnosis of FPHL is usually made clinically. However, histological evaluation using a 4 mm punch skin biopsy can be performed very rarely to confirm the diagnosis or to rule out other differential diagnoses of non-scarring alopecia, such as acute/chronic telogen effluvium (resting hair loss) and diffuse alopecia areata or early scarring hair loss, such as early central centrifugal cicatricial alopecia and fibrosing alopecia with a patterned distribution [28]. When needed, the skin biopsy should be taken from a representative site within the central scalp. Both temporal regions should be avoided as miniaturized hair follicles can present there independent of FPHL [28]. A punch biopsy of at least 4 mm size should be taken following the direction of the hair growth and reaching deeply into the level of subcutaneous tissue where the bulbs of anagen hair follicles usually reside. Horizontal sectioning of the biopsy is the standard technique used by many dermatopathologists as it allows a more accurate count of terminal and vellus-like hair follicles. A reduction of the terminal to vellus-like hair ratio from the normal of 9:1 to 1.9:1 – 2.2:1 is considered the hallmark of androgenetic alopecia. This might be associated with a reduction in the number of hairs per unit area to less than the normal of 240–400 hairs/cm2 or 30–50 hairs per 4 mm punch biopsy, a change that might not occur in the early stages of FPHL. A perifollicular lymphohistiocytic infiltrate and perifollicular fibrosis might occasionally be seen in androgenetic alopecia [28,32].
A screening blood work is usually needed to rule out the presence of other abnormalities that might cause telogen effluvium (resting hair loss) and unmask FPHL. These include serum ferritin, thyroid-stimulating hormone, vitamin D, and zinc. Iron deficiency might cause the hair follicle to enter telogen, causing telogen effluvium. In addition, it might also interfere with the efficacy of treatments used for FPHL [28]. Low serum ferritin is diagnostic for iron deficiency. However, the sensitivity of serum ferritin as a tool to diagnose low iron stores in patients with chronic inflammatory diseases, malignancy, or infections can be an issue, as ferritin is considered an acute phase reactant [28]. Measurement of both serum iron and total iron-binding capacity can be used as an alternative. Low serum iron and a high total iron-binding capacity can be expected in cases of iron deficiency. Hypothyroidism may also cause hair loss by inducing telogen effluvium. Moreover, zinc deficiency can cause hair loss, and vitamin D receptor-mutated animals and humans usually have alopecia [28,33].
Most women with FPHL have no underlying evidence of androgen excess [22,25]. Therefore, the decision to evaluate the patient’s serum androgen levels should be guided by the presence of relevant symptoms and signs. An early onset of FPHL can be a marker of a carrier state of a gene causing PCOS [34]. Moreover, the presence of concomitant signs and symptoms of hyperandrogenism such as hirsutism, moderate to severe or refractory resistant adult acne, irregular menses, infertility, or signs of insulin resistance, such as acanthosis nigricans and obesity, should warrant screening the patient for biochemical evidence of androgen excess. The most useful initial test is serum total testosterone level. Total testosterone assays measure free testosterone, albumin bound, and sex hormone-binding globulin (SHBG) bound testosterone. Although assessment of the free testosterone level has a more diagnostic yield as compared with that of total testosterone, its measurement is time consuming and costly [8]. Moreover, an excellent correlation between total and free testosterone has been described; and therefore, bioavailable testosterone can usually be predicted from the total testosterone level [8]. Measurement of DHEA-S should be ordered in cases where an androgen adrenal secreting tumor is suspected [8]. Testosterone levels greater than 2.5× normal or >150–200 ng/dL, or DHEA-S greater than 2× normal or >700 µg/dL in premenopausal or >400 µg/dL in postmenopausal women, should necessitate a radiological workup to rule out the presence of androgen-secreting tumors of ovaries or adrenals [35]. An early morning serum 17-OH progesterone during the follicular phase of the cycle (days 1–14) can be used as a screening test for nonclassic CAH [36]. Prolactin level can be ordered in cases of galactorrhea or increased serum testosterone level [8]. Further evaluation by an endocrinologist will be needed in patients with high values of testosterone, DHEA-S, prolactin, or 17-OH progesterone [28].
Treatment
Successful treatment of FPHL depends on meeting realistic expectations made by patients, as almost all available treatment modalities are aimed at slowing down the hair loss and at most arresting it. Hence, no dramatic changes in hair thickness should be expected. However, stimulating growth and thickening of pre-existing hair can be achieved in some patients using drug treatments. Starting medications early and combining more than one medication together might be the key for better results. On the other hand, hair transplantation surgery can be very effective, which, when performed and combined with the use of appropriate medicines, can result in superior results compared with using medications alone.
Considering the mode of action, treatments used for FPHL can be divided into two categories: androgen independent and androgen dependent. Other miscellaneous groups of treatments will also be discussed.
Androgen-independent therapies
Minoxidil
There are two different concentrations for topical minoxidil solution commercially available: 2% and 5%. This is in addition to the recently approved 5% foam preparation. Minoxidil 2% solution (twice daily) and 5% foam are the only concentrations approved by the United States Food and Drug Administration and other countries’ regulatory equivalents for treating FPHL. However, the 5% solution is increasingly being used off-label for FPHL. Minoxidil sulfate, minoxidil’s active metabolite, works by opening the cellular potassium channels, leading to impaired entry of calcium into the hair follicle cells, thus decreasing epidermal growth factors and enhancing hair growth. Minoxidil was also found to prolong the anagen phase of hair growth, transforming vellus and vellus-like hairs into terminal hairs [1,37]. Minoxidil can either arrest hair loss or induce mild to moderate hair regrowth in 60% of women [28,38]. A randomized, placebo-controlled trial comparing the efficacy of topical 5% solution with topical 2% and placebo in FPHL patients demonstrated statistically significant increased hair growth in both 5% and 2% groups over the placebo group. However, although not statistically significant, the 5% solution seemed to work better than the 2% solution [38]. Side effects include contact dermatitis in 6.5% of patients and facial hypertrichosis in 3–5% of patients [37]. Tachycardia and hypotension can also be seen in 1 out of every 1000 patients [1]. Switching to the lower concentration of 2% solution or to the 5% foam preparation can help to alleviate most of the side effects.
Minoxidil should be avoided during pregnancy or breast feeding. Minoxidil is considered as pregnancy category C by the US Food and Drug Administration (FDA). However, a one-year prospective study showed no increase in cardiovascular events or adverse pregnancy outcomes among patients on topical minoxidil versus controls, but there have been scattered reports of fetal malformations [37]. Minoxidil can be used for FPHL with or without hyperandrogenism. It can also be used alone or in combination with other medications, such as antiandrogens. One mL of minoxidil should be applied and spread over the frontal and crown region of a dry scalp twice daily for at least 6 months before judging the efficacy. When effective, minoxidil should be used for life, as discontinuing minoxidil will cause the responsive hair follicles to fall out. It is the author’s opinion that minoxidil should be included in any treatment regimen of FPHL and should be considered the first-line treatment.
Androgen-dependent therapies
Two groups of antiandrogen agents are recognized. The first group comprises the classic antiandrogen agents, including spironolactone, cyproterone acetate, and flutamide, which are capable of blocking cytoplasmic androgen receptors. The second group includes peripheral antiandrogen agents, such as finasteride and dutasteride, which work by blocking the conversion of testosterone to DHT in the hair follicles. None of the above groups are approved for use in FPHL and they are uncommonly used in North America, unlike in Europe where they are used more commonly.
Spironolactone
Spironolactone is a potassium-sparing diuretic and a synthetic steroid structurally related to aldosterone. It exerts its antiandrogen effects by competitively blocking the DHT cytoplasmic receptors and in addition it has a weak inhibitory effect on androgen synthesis. Although many studies have demonstrated its efficacy in the treatment of FPHL, especially when associated with other signs of hyperandrogenism, such as hirsutism and acne, spironolactone is being used off-label to treat FPHL [39,40]. Most women will require a minimum of 200 mg per day for at least 6 months [29]. Tolerable side effects include lethargy, nausea, breast engorgement, and menorrhagia. It can also induce hyperkalemia and hypotension, warranting a periodic monitoring of potassium levels [25]. Spironolactone is in pregnancy category D and might cause feminization of a male fetus. Therefore, it is contraindicated for women of childbearing age without the use of appropriate contraception methods. Low-dose oral contraceptive pills (OCPs) should be helpful to prevent pregnancy and to reduce the menorrhagia.
Cyproterone acetate
Cyproterone acetate is an androgen receptor blocker. It can also decrease testosterone levels by suppressing luteinizing hormone and follicle-stimulating hormone release [29]. Improvement of FPHL was demonstrated when cyproterone acetate was used alone or in combination with ethinylestradiol or spironolactone [37]. It can be used for FPHL with or without hyperandrogenism. The most useful dose is 100 mg on days 5–15 combined with 50 µg ethinylestradiol on days 5–25 of the menstrual cycle [28]. The hair loss requires a higher dose than the 2 mg daily dose found in the combined estrogen–progestin OCP called Diane (2 mg cyproterone acetate days 5–15 and 50 µg of ethinylestradiol days 5–25 of menstrual cycle) that can be used for hirsutism [25]. Fatigue, edema, loss of libido, weight gain, nausea, and depression are the most recognized side effects. Moreover, higher doses have been reported to induce hepatitis [25]. Cyproterone acetate can cause feminization of the male fetus. Therefore, its use should be accompanied by the use of appropriate OCPs.
Flutamide
Flutamide is a nonsteroidal androgen receptor blocker used primarily for prostate cancer. A low dose of the drug (62.5 mg per day) was found to improve FPHL in hyperandrogenic women and to be well tolerated at the same time. A dose-dependent risk of severe hepatotoxicity has made this medication less attractive in dermatology [40,41].
Finasteride
Finasteride works by inhibiting type II 5α-reductase. This lowers serum and scalp levels of DHT [37]. Although one double-blind controlled trial failed to show any statistical difference in efficacy in a group of postmenopausal women who used finasteride 1 mg for one year compared with a placebo, another study has shown the efficacy of oral daily dosage of finasteride 5 mg in treating a group of normoandrogenic pre- and postmenopausal women with FPHL [42]. Moreover, finasteride 2.5 mg per day was effective in 62% of a group of premenopausal normoandrogenic females with FPHL while taking OCPs containing drospirenone and ethinylestradiol [31]. Finasteride 1.25 mg per day was also beneficial in stabilizing or improving FPHL in one case series of four premenopausal women with hyperandrogenism [43]. Therefore, considering the uncertain role of increased androgens versus excessive activity of 5α-reductase enzyme in the pathogenesis of FPHL as compared with AGA-M, there will be some variability in the proportion of female patients who would respond to finasteride and in the minimum effective dose that is needed to induce a clinical improvement. It seems that a higher dose of finasteride as compared with the regular 1–1.25 mg daily dose for men will be needed to manage FPHL [37]. Finasteride is in pregnancy category X. Therefore, its use is contraindicated in premenopausal women without appropriate contraceptive methods as it can cause feminization of a male fetus. Pregnant women are even instructed not to handle crushed or broken pills given that possible risk [37]. Finasteride is metabolized in the liver, but it does not affect the cytochrome P450-linked drug metabolizing enzymes. Thus, no clinically recognized drug interactions should be expected. A safer approach is to avoid using it for patients with known liver abnormalities [37]. Longer and better controlled trials may be needed to confirm finasteride’s safety and efficacy in women.
Dutasteride
Dutasteride exerts a dual action by blocking both types of 5α-reductase enzyme, I and II. It is also one hundred times and three times as potent as finasteride at inhibiting type I and II 5α-reductase enzymes, respectively. Given these enhanced effects on 5α-reductase enzymes, it was found that dutasteride can reduce serum DHT level by 90% as compared with the 70% reduction rate seen with finasteride [37]. One case report showed that dutasteride 0.5 mg per day was effective at reversing FPHL in a woman who failed to respond to finasteride [37]. Dutasteride is not yet approved for use in pattern hair loss in either males or females.
Miscellaneous therapies
Hair transplantation
Hair transplantation surgery is considered to be an important option for females in whom medical therapy alone has failed or induced only a partial response. It is an outpatient procedure which is performed under local anesthesia. It classically involves dividing a harvested occipital strip into individual hair follicles in a process called follicular unit transplantation (FUT). The resulting individual hair follicles are then strategically placed in the recipient’s sites, which are usually located in the frontal scalp [31].
Camouflage
A variety of camouflaging topical sprays, powders, or keratin fibers are available. Their use can be helpful for patients as they usually give a temporary denser look to the scalp hair by sticking to pre-existing hair fibers.
Cranial prostheses (wigs, hairpieces)
Different types of cranial prosthesis are available, ranging from off-the-shelf synthetic wigs to custom-made real hair wigs that are designed to cover the entire scalp. Localized areas of hair loss can be covered using specially designed hairpieces. Moreover, hair extensions are an alternative option to add more volume or length to the hair.
Hirsutism
Hirsutism is defined as excessive terminal hair growth in females growing in typical male distribution. It only affects the androgen-dependent hair follicles. Hirsutism should be differentiated from hypertrichosis in which there is excessive growth of androgen-independent vellus hair in nonsexual areas. Hirsutism affects 5–10% of women of reproductive age [5]. The modified Ferriman–Gallwey scoring system is regarded as the standard for assessing severity of hirsutism in clinical investigations [8]. In this scoring system, each of nine areas most sensitive to androgens is assigned a score from 0 (no hair) to 4 (frankly virile), and then the sum of the scores provides a hirsutism score. Although no value is defined as a threshold to diagnose hirsutism, a score of 6–8 is probably an acceptable value [44]. Mild hirsutism is defined as a score of 8 to 15. Moderate to severe hirsutism is associated with a score of >15 [11] (Figure 2.2).
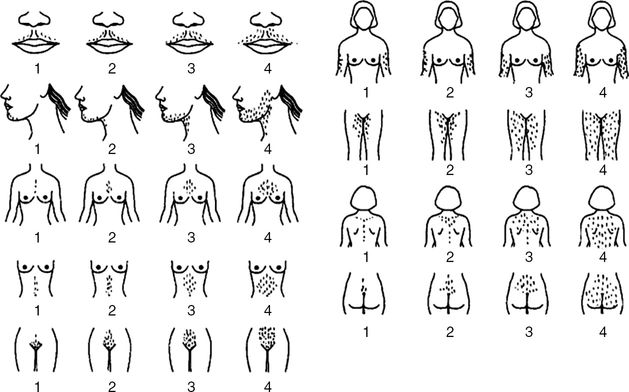
Modified Ferriman–Gallwey scoring system. Each one of the nine body areas most sensitive to androgens is assigned a score from 0 (no hair) to 4 (frankly virile). These scores are summed to give a hirsutism score. Reprinted with permission from R. Azziz (Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update 2010;16:51–64.). Copyright Oxford University Press, 2010.
Androgens and mainly testosterone, as discussed earlier, play a major role in inducing hair follicles to grow thicker, longer, and darker hairs. This is invariably true in androgen-sensitive areas such as the face, chest, lower abdomen, pubis, and axillae. Moreover, hirsutism is regarded as the most common complaint associated with androgen excess, with most women who have at least twice the normal upper limit of androgens developing some degree of hirsutism [8]. Despite that, severity of hirsutism is usually determined by the subject’s individual follicular androgen sensitivity rather than the actual androgen level [11].
Causes of hirsutism
Hirsutism has always been considered as a sign of androgen excess. Therefore, different possible causes of hyperandrogenism will be outlined here. Causes will be discussed under four headings: ovarian, adrenal, iatrogenic, and idiopathic.
Ovarian causes
Polycystic ovary syndrome (PCOS)
PCOS is regarded as the most common cause of hirsutism in woman presenting with signs of androgen excess, with a prevalence ranging between 57% and 82% [8,45,46]. Hirsutism secondary to PCOS usually starts around puberty and progressively and slowly worsens over time. Other signs of androgen excess can accompany the hirsutism, including irregular cycles, infertility, weight gain, acne, and hair loss in a pattern distribution. Associated insulin resistance can give rise to acanthosis nigricans.
Features of PCOS are believed to occur as a consequence of chronic anovulation caused by a wide variety of factors rather than being attributed to a specific endocrinological disorder [8]. Therefore, a history of ovulatory dysfunction (such as amenorrhea and oligomenorrhea) of pubertal onset is considered as the most prominent feature of PCOS. In fact, the history of cyclic predictable menses makes the diagnosis of PCOS unlikely. The Androgen Excess Society (AES) has suggested criteria for the diagnosis of PCOS. Clinical or biochemical signs of hyperandrogenism, such as hirsutism and/or hyperandrogenemia, evidence of ovarian dysfunction either clinically in a form of oligo-anovulation or radiological evidence of polycystic ovaries, and lastly the exclusion of other causes of androgen excess or related disorders were all included in the AES diagnostic criteria of PCOS [47].
Hyperthecosis
This refers to the hyperplasia of luteinized theca cells in the ovarian stroma. Apart from a greater degree of hyperandrogenism, the symptoms of hyperthecosis are very similar to those of PCOS [8]. Hyperthecosis is regarded as a severe variant of PCOS [47].
Ovarian neoplasms
Rapidly progressing hirsutism, which might be accompanied by other signs of virilization, should warrant searching for androgen-secreting neoplasms. Androgen-secreting tumors of ovaries and adrenals are the cause of androgen excess in 0.2% of cases [47]. While most androgen-secreting neoplasms arise from ovaries, less than 1% of ovarian neoplasms secrete androgens. Sertoli–Leydig and hilus cell tumors are the most common testosterone-secreting ovarian tumors. The former tumor occurs during the reproductive years, while the latter affects postmenopausal women. These ovarian tumors secrete large quantities of testosterone or its precursor androstenedione, resulting in a total testosterone values exceeding 200 ng/dL [35,47].
Adrenal causes
Congenital adrenal hyperplasia (CAH)
Females with classic CAH are usually recognized at birth with ambiguous genitalia. On the other hand, the nonclassic form (late onset) CAH presents usually at or after puberty with hirsutism and menstrual irregularities. CAH is responsible for 1–2% of hirsutism cases [45]. 21-Hydroxylase deficiency is the most common underlying cause in both types of CAH. The 21-hydroxylase enzyme is responsible for converting 17α-hydroxyprogesterone to 11-deoxycortisol. The resulting low glucocorticoid concentration stimulates adrenocorticotropic hormone (ACTH) production, which in turn causes adrenal hyperplasia and an increase in androgen production [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


