Feature
2D-TVS
Serosal contour of the uterus
Uterus often globally enlarged
Definition of lesion
Ill-defined in diffuse adenomyosis (adenomyoma may be well-defined)
Symmetry of uterine walls
Anterior-posterior myometrial asymmetry
Outline
Ill-defined
Shape
Ill-defined
Contour
Irregular or ill-defined
Rim
No rim
Shadowing
No edge shadows, fan shaped shadowing, linear hypoechoic striation
Echogenicity
Non-uniform
Presence of intramyometrial:
Mixed echogenicity
Cyst
Hyper-echogenic islands
Subendometrial echogenic lines
Buds
Vascularity
Translesional flow
Diffuse minimal or few vessels
Endometrial rim
Irregular or ill-defined
Distorted or imprinted
3D-TVS
JZ thickness
Thickened JZ:
Maximum JZ thickness (JZmax) > 6–8 mm
Ratio of JZ (JZmax/total myometrial wall thickness) ≥50 %
Difference (JZmax) − (JZmin) = JZdif ≥4 mm
JZ regularity
Irregular or ill-defined
Distorted
JZ interruption
Interrupted
Infiltration of the JZ by hyperechoic endometrial tissue
a globally enlarged uterus: the fundus of the uterus appears enlarged (Fig. 9.1)
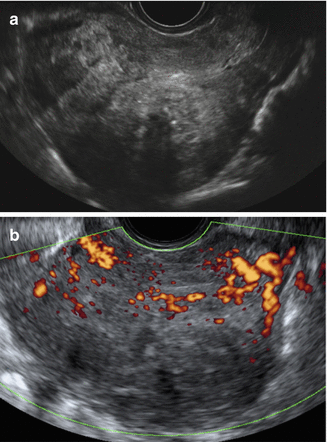
Fig. 9.1
Ultrasound images of a uterus with adenomyosis. (a) Gray scale image showing globally enlarged uterus unrelated to leiomyoma with irregular myometrial echotexture with hyperechoic irregular myometrial areas and small anechoic areas. Note the ill-defined endometrial stripe. (b) Power Doppler image showing diffusely spread vessels without the circular flow along a capsule that is typical for leiomyoma
asymmetrically enlarged uterus (for example anterior wall thicker than posterior wall or vice versa) unrelated to leiomyoma (Figs. 9.1 and 9.2)
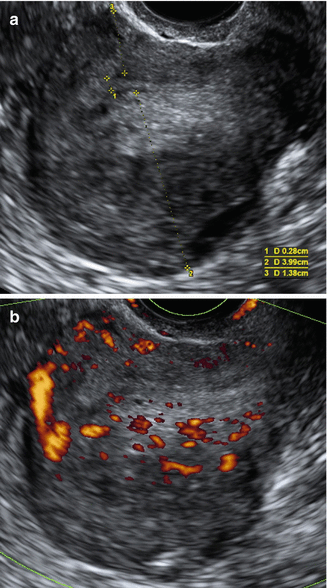
Fig. 9.2
Ultrasound images of a uterus with adenomyosis. (a) Gray scale image showing asymmetrically thickened, posterior wall (2) is thicker than anterior wall posterior uterine wall 3, with inhomogeneous, irregular myometrial echotexture due to hyperechoic and small cystic anechoic areas. Endometrial thickness (1). (b) Power Doppler image showing diffusely spread small vessels
round cystic area within the myometrium (Fig. 9.3)
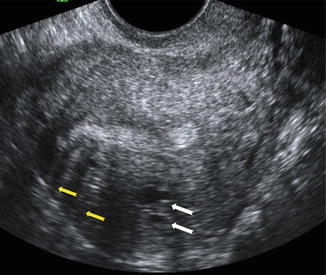
Fig. 9.3
Ultrasound image of a uterus with adenomyosis. Note the round cystic anechoic areas (white arrows) in the myometrium below the endometrium and hypoechoic linear striation (yellow arrows)
inhomogeneous, irregular myometrial echo texture in an indistinctly defined myometrial area with decreased or increased echogenicity; hyper-echogenic islands, subendometrial lines and buds (Fig. 9.4)
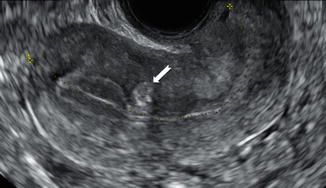
Fig. 9.4
Ultrasound image of a uterus with a hyperechoic buds (white arrows) in the myometrium beneath the endometrium
myometrial hypoechoic linear striations seen as a radiating pattern of thin acoustic shadows not arising from echogenic foci or leiomyoma (fan shaped shadowing) (Fig. 9.3)
indistinct, fuzzy endometrial-myometrial border (ill-defined endometrial stripe) (Figs. 9.1 and 9.2)
presence of diffuse minimal vascularity seen as diffusely spread of small vessels which do not have the normal course of the arcuate and radial arteries inside the myometrium (Figs. 9.1, 9.2, 9.5, and 9.6)
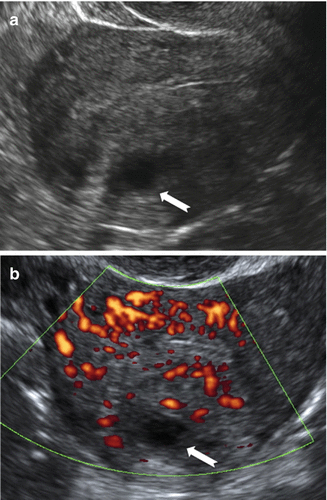
Fig. 9.5
Ultrasound images of a uterus with focal cystic adenomyosis of the posterior wall. (a) Gray scale image showing a cystic anechoic area in the myometrium of the posterior wall (white arrow). (b) Power Doppler image showing diffusely spread vessels around the cystic area (white arrow)
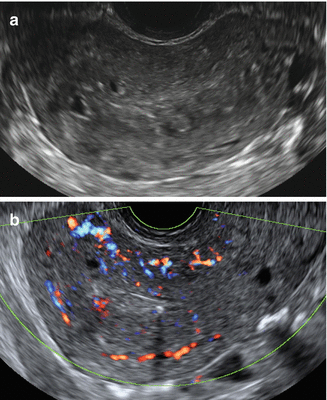
Fig. 9.6
Ultrasound images of a uterus with diffuse cystic adenomyosis of the uterus. (a) Gray scale image showing several small cystic anechoic areas and hyperechoic islands in the myometrium, note the ill-defined endometrial stripe. (b) Power Doppler image showing diffusely spread vessels in the affected myometrium
Moreover, a new interesting sign called question mark form of uteri was reported recently [14]. This is described when the corpus uterus is flexed backwards, the fundus of uteri is facing the posterior pelvic compartment and the cervix is directed frontally towards the urinary bladder (Fig. 9.7).
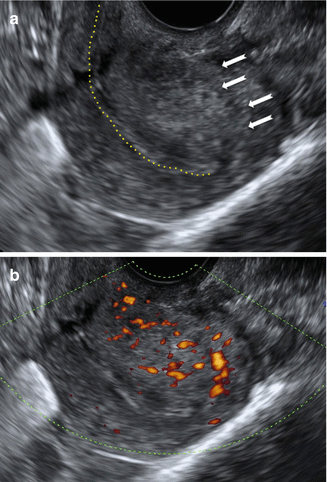

Fig. 9.7
Ultrasound images of a retroverted uterus with adenomyosis with the typical ‘question sign’. (a) Gray scale image showing asymmetrically thickened posterior uterine wall with abnormal echogenicity (white arrows). (b) Power Doppler image showing diffusely spread small vessels
Power Doppler can be used to distinguish myometrial cysts from blood vessels and to discriminate between leiomyomas and focal adenomyosis (Figs. 9.5 and 9.6). Uterine leiomyomas feature circular flow along the myoma capsule, while localized adenomyosis and adenomyomas are characterized by diffusely spread vessels inside the lesions (Figs. 9.1, 9.2, and 9.8). Reinhold et al. reported that 2D-TVS had a sensitivity of 80–86 %, specificity of 50–96 %, and overall accuracy of 68–86 % for diagnosing diffuse adenomyosis [2]. However, 2D-TVS can yield equivocal result in the case of focal adenomyosis and in the presence of coexistent fibroids [1, 5]. A recent meta-analysis of 14 trials involving 1985 participants, reported the sensitivity and specificity of ultrasound diagnosed adenomyosis to be as high as 82.5 and 84.6 %, respectively [15].
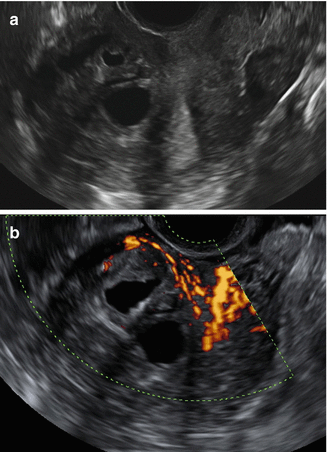

Fig. 9.8
Ultrasound images of a uterus with an anterior cystic adenomyoma. Gray scale image showing inhomogeneous, irregular myometrial echotexture with cystic areas (a). Power Doppler image showing more circular vessels in the hypertrophic myometrium surrounding the cystic areas (b)
Usually the diagnosis adenomyosis is made on histological examination of a uterus following a hysterectomy. The histological frequency of adenomyosis ranges from 5 to 70 % according to reported series. The wide variation is affected by the histological criteria and the number of sections examined. The majority of previous studies reporting the accuracy of 2D-TVS diagnosis of adenomyosis have assessed populations of women who underwent hysterectomy [1, 15, 16]. These included mainly women with severe symptoms who were more likely to have adenomyosis compared to the general population, and it is likely that the prevalence of adenomyosis in these studies is an overestimate.
Furthermore, the 2D-TVS findings are more likely to appear in advanced stages of the disease. Most of the studies utilising TVS required the presence of at least two or three of ultrasound features for the diagnosis of adenomyosis [8, 9, 17–19]. But the presence of only one of the typical TVS features of adenomyosis raises some concern especially in young women (Fig. 9.9).
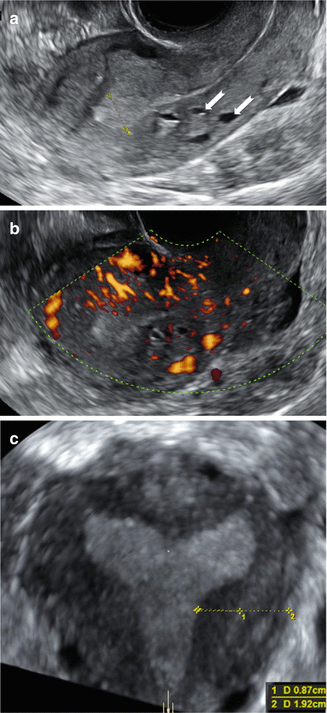

Fig. 9.9
Ultrasound images of a uterus in a 25 year old woman with cystic areas only in the posterior wall (white arrows). Gray scale image (a) and Power Doppler image (b) showing diffusely spread small vessels surrounding the cystic areas. Coronal section (c) obtained by 3D ultrasound of the same uterus showing a thickened JZ
Targeted ultrasound guided biopsies have been proposed in an attempt to correlate histological findings to ultrasound features of adenomyosis in those younger women who will not undergo hysterectomy [20–22]. Recently, Nam and Lyu, performed abdominal ultrasound-guided transvaginal myometrial core needle biopsy in 1032 premenopausal women aged 22–53 years who had 2D-TVS findings suggestive for adenomyosis [22]. They reported a 92.26 % concordance rate between the transvaginal myometrial core needle biopsy and ultrasonographic diagnoses of adenomyosis [22].
The severity of adenomyosis is difficult to express in quantitative terms as the lesions are often poorly defined and may be disseminated throughout different parts of the myometrium. The number of different morphological features in an individual woman has been proposed as an indirect semi-quantitative measure of severity of adenomyosis [8, 19].
Tomassetti et al. defined a number of factors encouraging the development of adenomyosis including a history of spontaneous miscarriage, curettage, hysteroscopic resection of the endometrium, uterine myomectomy, caesarean section and the use of tamoxifen [23]. Furthermore, there are a number of studies that reported a correlation between ultrasound findings of adenomyosis and symptoms like menometrorrhagia or dysmenorrhea, infertility and multiparity has been shown [24–26]. Therefore the association with symptoms could improve the diagnostic accuracy of ultrasound features for adenomyosis [8, 19, 24].
Adenomyosis seems also to be associated with endometriosis [17, 27, 28]. According to some authors, adenomyosis seems to share pathogenic mechanisms as well as similar symptoms (menometrorrhagia dysmenorrhea, dyspareunia, abnormal uterine bleeding and infertility), with endometriosis [27, 29, 30]. Knowing the prevalence of adenomyosis in a population with endometriosis helps in evaluating the likely contribution of adenomyosis to their symptoms.
Di Donato et al [2014] reported recently on a series of patients undergoing surgery for endometriosis a prevalence of adenomyosis diagnosed by ultrasound of 21.8 %. This prevalence is slightly higher than the prevalence (20.9 %) reported by Naftalin who evaluated patients attending a general gynecological ultrasound unit [28]. An interesting feature is the strong association between deep infiltrating endometriosis and adenomyosis reported in some recent studies [17, 31]. In a recent study, Lazzeri et al. confirmed the strong association between adenomyosis diagnosed by transvaginal ultrasound and endometriosis. The incidence of adenomyosis in patients affected by deep infiltrating endometriosis was 48.7 % [17]. Di Donato et al. found that the ultrasound ‘question mark sign’ of the retroverted fixed uterus, was strongly related to posterior deep infiltrating endometriosis (Fig. 9.10) [14].
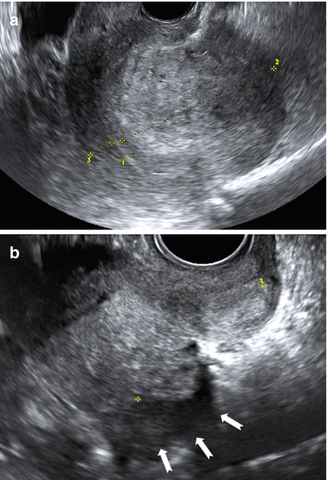

Fig. 9.10




Ultrasound images of a retroverted uterus with adenomyosis with the typical ‘question sign’. (a) Grayscale image showing asymmetrically thickened posterior uterine wall (2) with abnormal echogenicity, (1 endometrila thickness, 3 thicknes of the anterior uterine wall). (b) Posterior deep infiltrating lesions (white arrows) involving the adenomyotic myometrium and infiltrating the rectal wall posteriorly
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


