65 Acute and Chronic Renal Failure
Acute Renal Failure
Etiology and Pathogenesis
Renal
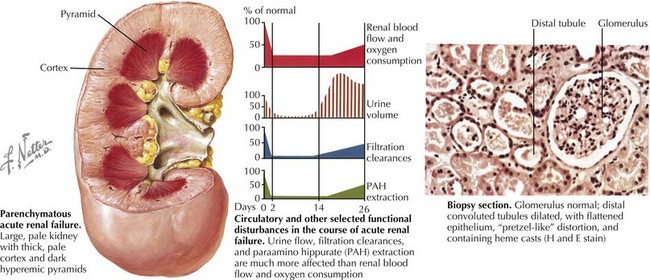
AKI (intrinsic renal disease) is the result of disorders that involve the renal vascular, glomerular, or tubular–interstitial pathology. Acute tubular necrosis (ATN) results from ischemia caused by decreased renal perfusion or injury from tubular nephrotoxins (Figure 65-1). All causes of prerenal AKI can progress to ATN if renal perfusion is not restored or nephrotoxins are not withdrawn. Nephrotoxic AKI is mostly caused by toxic tubular injury by medications, including aminoglycosides, contrast agents, amphotericin B, chemotherapeutic agents (ifosfamide, cisplatin), and acyclovir. Toxic tubular injury can also be induced by the release of heme pigments, as it occurs from myoglobinuria caused by rhabdomyolysis and hemoglobinuria caused by intravascular hemolysis. Uric acid nephropathy and tumor lysis syndrome are causes of AKI in children with leukemia. During chemotherapy, a rapid breakdown of tumor cells causes increased release and subsequent excretion of uric acid, resulting in precipitation of uric acid crystals in the tubules and renal microvasculature. Hyperphosphatemia in tumor lysis syndrome results in precipitation of calcium phosphate crystals in the tubules. Acute interstitial nephritis most commonly results from hypersensitivity reactions to drugs, including penicillin analogs (e.g., methicillin), cimetidine, sulfonamides, rifampin, nonsteroidal antiinflammatory drugs, and proton pump inhibitors, but can also be idiopathic. Glomerulonephritis of any etiology (including those caused by vasculitis, systemic lupus erythematosus, or Goodpasture’s syndrome) may present with AKI, with postinfectious glomerulonephritis being the most common cause of AKI in this group. Rapidly progressive glomerulonephritis presents as the most severe degree of any form of glomerulonephritis and presents with AKI. Vascular causes of AKI include cortical necrosis (mostly caused by hypoxic or ischemic injury in newborns), renal artery or vein thrombosis, and hemolytic-uremic syndrome (HUS).
Clinical Presentation
Evaluation and Diagnosis
Serum Creatinine Concentration, Acute Kidney Injury Biomarkers, and Classification of Acute Kidney Injury
Fractional Excretion of Sodium
Renal Imaging
Renal ultrasonography should be performed in all children with AKI. It can document the presence of one or two kidneys, determine renal size (often enlarged in those with AKI) (see Figure 65-1), assess the renal parenchyma, and diagnose urinary tract obstruction or occlusion of the major renal vessels.
Prevention and Management
The basic principles of the general management of AKI are shown in Box 65-1.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree