8. Acid-Base Homeostasis and Oxygenation*
Amy M. Wood and M. Douglas. Jones Jr.
Examination of arterial blood gases and interpretation of acid-base balance are essential to proper diagnosis, management, and outcome in an ill neonate. 3,17,28 The measurement of arterial blood gases allows analysis of two interrelated but separate processes: acid-base homeostasis and oxygenation. 1,16,21 This chapter describes the parameters that designate these processes, their measurements, and the effects of proposed treatment on homeostasis. 1,28 Common abbreviations and their meanings are listed in Box 8-1.
BOX 8-1
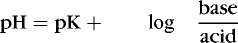
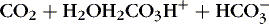
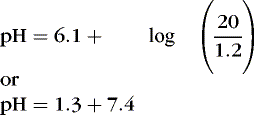
Get Clinical Tree app for offline access
A Alveolar
a Arterial
D Difference
F Fraction
I Inhalation, inspired
P Partial pressure (tension, driving force)
pH Negative log of hydrogen ion concentration
Combined Abbreviations
Pa o2Partial pressure of arterial oxygen
F io2Fraction of inspired oxygen
P io2Partial pressure of inspired oxygen
Components of arterial blood gases include (1) actually measured values (Pa o2, Pa co2, and pH) and (2) calculations from these values (oxygen saturation, bicarbonate concentration, and base excess). Some analyzer systems also estimate hemoglobin concentration. The pH, Pa co2, base excess, and bicarbonate components are used to assess acid-base homeostasis, 16,21,31 whereas Pa o2, saturation (Sa o2), and hemoglobin1,3 are used to assess adequacy of oxygenation (Table 8-1).
| Blood Gases | Values |
|---|---|
| pH | 7.35-7.45 |
| Paco 2 | 35-45 mm Hg |
 | 18-26 mEq/L |
| Base excess | (−5) to (+5) |
| Pao 2 | 60-80 mm Hg |
| O 2 saturation | 92%-94% |
PHYSIOLOGY
Acid-Base Homeostasis
To review basic chemistry, an acid is a hydrogen ion donor and a base is a hydrogen ion receptor. The pH refers to the concentration of hydrogen ions [H +] in blood and reflects the acid-base balance in blood. 31 The quantity of hydrogen ions is minute, approximately 0.0000001 mole/L. Therefore the negative log of the hydrogen ion concentration is used to define pH and create a positive, workable number (pH = 7) (Equation 1). A pH of 7 represents a neutral solution, a pH of less than 7 represents acidity, and a pH greater than 7 represents alkalinity:
(1)
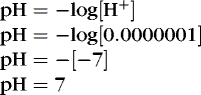
The Henderson-Hasselbalch equation describes pH as equal to a constant (pK) plus the logarithm of the ratio of the base-to-acid concentration (Equation 2). 3,17 Thus if there is an increase in the concentration of hydrogen ions (reflected in the denominator), the blood pH value decreases and acidemia results. Conversely, if there is less acid or more base, pH increases and alkalemia results. 3
(2)
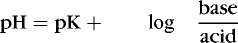
The first step in determining acid-base homeostasis is measurement of pH. Normal human pH is between 7.35 and 7.45. Acidemia and acidosis are often used interchangeably, but strictly speaking, pH of less than 7.35 is acidemia and the process that caused it is acidosis; a pH of greater than 7.45 is alkalemia and the process that caused it is alkalosis. 17Arterial carbon dioxide (Pa co2) and bicarbonate [ 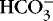 ] values represent the two main components of acid-base homeostasis: (1) respiratory contribution (Pa co2) controlled by alveolar ventilation,1,8,11and (2) nonrespiratory or metabolic contribution controlled primarily by renal excretion, retention, or production of [
] values represent the two main components of acid-base homeostasis: (1) respiratory contribution (Pa co2) controlled by alveolar ventilation,1,8,11and (2) nonrespiratory or metabolic contribution controlled primarily by renal excretion, retention, or production of [ 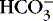 ]. 1,15,26Other factors that affect nonrespiratory components of acid-base balance cause a change in [
]. 1,15,26Other factors that affect nonrespiratory components of acid-base balance cause a change in [ 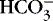 ] ; thus [
] ; thus [ 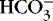 ] is an indicator of the nonrespiratory component. 15,19,26
] is an indicator of the nonrespiratory component. 15,19,26
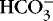 ] values represent the two main components of acid-base homeostasis: (1) respiratory contribution (Pa co2) controlled by alveolar ventilation,1,8,11and (2) nonrespiratory or metabolic contribution controlled primarily by renal excretion, retention, or production of [
] values represent the two main components of acid-base homeostasis: (1) respiratory contribution (Pa co2) controlled by alveolar ventilation,1,8,11and (2) nonrespiratory or metabolic contribution controlled primarily by renal excretion, retention, or production of [ 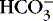 ]. 1,15,26Other factors that affect nonrespiratory components of acid-base balance cause a change in [
]. 1,15,26Other factors that affect nonrespiratory components of acid-base balance cause a change in [ 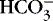 ] ; thus [
] ; thus [ 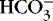 ] is an indicator of the nonrespiratory component. 15,19,26
] is an indicator of the nonrespiratory component. 15,19,26RESPIRATORY CONTRIBUTION
Carbon dioxide is produced by each cell as a product of metabolism. 1,23 As carbon dioxide is produced, it dissolves in intracellular fluid and can be measured as the partial pressure (P) of the dissolved gas (CO 2). As the pressure of the dissolved gas increases inside the cell, carbon dioxide moves out of the cell into the blood. Blood transports dissolved carbon dioxide (some combined with hemoglobin as carboxyhemoglobin, most as bicarbonate) to the lung, where the partial pressure in the pulmonary capillary is greater than in the alveoli, 21 causing carbon dioxide to move into the alveoli. Ventilation is the only method of removing carbon dioxide. The amount of carbon dioxide in the blood is the net result of the body’s metabolism (production) and alveolar ventilation (clearance). Because metabolism does not change greatly and CO 2 diffuses easily across membranes, the only clinically important limitation to CO 2 removal is at the lungs. Thus Pa co2 reflects alveolar ventilation.3,8,11,17
In the red blood cell, the enzyme carbonic anhydrase promotes combination of a fraction of dissolved CO 2 with water to form carbonic acid (H 2CO 3), which then dissociates into a hydrogen ion [H +] and a bicarbonate ion [ 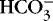 ] 1,23:
] 1,23:
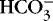 ] 1,23:
] 1,23:(3)
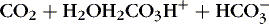
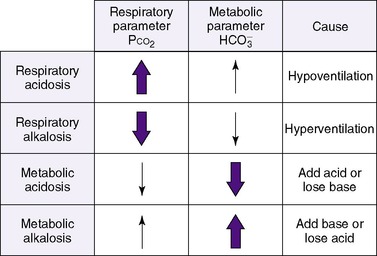
Therefore an increase in Pa co2 causes pH to fall. Hypoventilation causes an increase in carbon dioxide. This is called respiratory acidosis because the respiratory system is responsible for regulating Pa co2 as the lung regulates the amount of carbon dioxide in the body.8 A decrease in Pa co2 results in less acid in the blood and causes pH to rise. A pathophysiologic process that causes hyperventilation and reduces dissolved carbon dioxide is known as respiratory alkalosis.
NONRESPIRATORY (METABOLIC) CONTRIBUTION
Nonrespiratory (metabolic) derangements can also disturb acid-base homeostasis. Normal metabolism produces hydrogen ions. Blood pH is maintained within normal limits by renal mechanisms for excreting hydrogen ions. Increased production of [H +] may occur in conditions such as shock with poor perfusion of the gastrointestinal system or genetically determined aberrations of metabolism.27 The hydrogen ions produced must be eliminated to avoid a fall in blood pH. Derangements also occur when hydrogen ions are lost (e.g., in gastric juice) or when bicarbonate is lost (e.g., in diarrheal fluid or ileostomy drainage). 28
Authorities differ as to the best way to describe nonrespiratory derangements in acid-base status. The traditional approach relies on measurement of pH, P co2 and [ 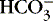 ]. The alternative is description of acid-base status in terms of (1) strong ions (Na +, K +, Ca 2+, Mg 2+, Cl −), strong because they remain dissociated at body pH, and (2) weak acids (hemoglobin, albumin, inorganic phosphate), weak because they are partially dissociated at body pH. Blood pH in this conceptualization is a function of the difference between strong cations and strong anions, the strong ion difference (SID). As an example, the alkalosis associated with loss of gastric fluid would be described exclusively in terms of loss of [Cl −] with loss of [H +] making no independent contribution to the resulting alkalemia. Advocates maintain that measurement of SID leads to greater understanding of the causes of nonrespiratory and mixed acid-base derangements. 7,12,14,23 Others favor staying with the traditional bicarbonate-center model. 6,18 The present discussion focuses on the traditional bicarbonate-centered approach. Readers are referred to recent reviews for comparisons of the two methods. 14,18
]. The alternative is description of acid-base status in terms of (1) strong ions (Na +, K +, Ca 2+, Mg 2+, Cl −), strong because they remain dissociated at body pH, and (2) weak acids (hemoglobin, albumin, inorganic phosphate), weak because they are partially dissociated at body pH. Blood pH in this conceptualization is a function of the difference between strong cations and strong anions, the strong ion difference (SID). As an example, the alkalosis associated with loss of gastric fluid would be described exclusively in terms of loss of [Cl −] with loss of [H +] making no independent contribution to the resulting alkalemia. Advocates maintain that measurement of SID leads to greater understanding of the causes of nonrespiratory and mixed acid-base derangements. 7,12,14,23 Others favor staying with the traditional bicarbonate-center model. 6,18 The present discussion focuses on the traditional bicarbonate-centered approach. Readers are referred to recent reviews for comparisons of the two methods. 14,18
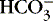 ]. The alternative is description of acid-base status in terms of (1) strong ions (Na +, K +, Ca 2+, Mg 2+, Cl −), strong because they remain dissociated at body pH, and (2) weak acids (hemoglobin, albumin, inorganic phosphate), weak because they are partially dissociated at body pH. Blood pH in this conceptualization is a function of the difference between strong cations and strong anions, the strong ion difference (SID). As an example, the alkalosis associated with loss of gastric fluid would be described exclusively in terms of loss of [Cl −] with loss of [H +] making no independent contribution to the resulting alkalemia. Advocates maintain that measurement of SID leads to greater understanding of the causes of nonrespiratory and mixed acid-base derangements. 7,12,14,23 Others favor staying with the traditional bicarbonate-center model. 6,18 The present discussion focuses on the traditional bicarbonate-centered approach. Readers are referred to recent reviews for comparisons of the two methods. 14,18
]. The alternative is description of acid-base status in terms of (1) strong ions (Na +, K +, Ca 2+, Mg 2+, Cl −), strong because they remain dissociated at body pH, and (2) weak acids (hemoglobin, albumin, inorganic phosphate), weak because they are partially dissociated at body pH. Blood pH in this conceptualization is a function of the difference between strong cations and strong anions, the strong ion difference (SID). As an example, the alkalosis associated with loss of gastric fluid would be described exclusively in terms of loss of [Cl −] with loss of [H +] making no independent contribution to the resulting alkalemia. Advocates maintain that measurement of SID leads to greater understanding of the causes of nonrespiratory and mixed acid-base derangements. 7,12,14,23 Others favor staying with the traditional bicarbonate-center model. 6,18 The present discussion focuses on the traditional bicarbonate-centered approach. Readers are referred to recent reviews for comparisons of the two methods. 14,18A fall in blood [ 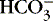 ] might indicate that bicarbonate, a base and therefore a hydrogen ion acceptor, has been used up by the addition of [H +]. As shown inEquation 3, a change in [
] might indicate that bicarbonate, a base and therefore a hydrogen ion acceptor, has been used up by the addition of [H +]. As shown inEquation 3, a change in [ 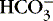 ] might also reflect a change in Pco2. This difficulty is overcome in blood gas analyzers by correcting the P co2 (graphically) to 40 mm Hg, yielding a “standard bicarbonate” concentration.23The standard bicarbonate concentration and the buffering properties of hemoglobin are combined in the concept of base excess (BE). A positive value suggests a deficit of fixed (i.e., not volatile as with H 2CO 3) acid or an excess of base; a negative value indicates an excess of fixed acid or a deficit of base.23An abnormality of the standard bicarbonate concentration or base excess indicates a process of nonrespiratory (metabolic) alkalosis15or nonrespiratory (metabolic) acidosis.26
] might also reflect a change in Pco2. This difficulty is overcome in blood gas analyzers by correcting the P co2 (graphically) to 40 mm Hg, yielding a “standard bicarbonate” concentration.23The standard bicarbonate concentration and the buffering properties of hemoglobin are combined in the concept of base excess (BE). A positive value suggests a deficit of fixed (i.e., not volatile as with H 2CO 3) acid or an excess of base; a negative value indicates an excess of fixed acid or a deficit of base.23An abnormality of the standard bicarbonate concentration or base excess indicates a process of nonrespiratory (metabolic) alkalosis15or nonrespiratory (metabolic) acidosis.26
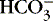 ] might indicate that bicarbonate, a base and therefore a hydrogen ion acceptor, has been used up by the addition of [H +]. As shown inEquation 3, a change in [
] might indicate that bicarbonate, a base and therefore a hydrogen ion acceptor, has been used up by the addition of [H +]. As shown inEquation 3, a change in [ 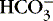 ] might also reflect a change in Pco2. This difficulty is overcome in blood gas analyzers by correcting the P co2 (graphically) to 40 mm Hg, yielding a “standard bicarbonate” concentration.23The standard bicarbonate concentration and the buffering properties of hemoglobin are combined in the concept of base excess (BE). A positive value suggests a deficit of fixed (i.e., not volatile as with H 2CO 3) acid or an excess of base; a negative value indicates an excess of fixed acid or a deficit of base.23An abnormality of the standard bicarbonate concentration or base excess indicates a process of nonrespiratory (metabolic) alkalosis15or nonrespiratory (metabolic) acidosis.26
] might also reflect a change in Pco2. This difficulty is overcome in blood gas analyzers by correcting the P co2 (graphically) to 40 mm Hg, yielding a “standard bicarbonate” concentration.23The standard bicarbonate concentration and the buffering properties of hemoglobin are combined in the concept of base excess (BE). A positive value suggests a deficit of fixed (i.e., not volatile as with H 2CO 3) acid or an excess of base; a negative value indicates an excess of fixed acid or a deficit of base.23An abnormality of the standard bicarbonate concentration or base excess indicates a process of nonrespiratory (metabolic) alkalosis15or nonrespiratory (metabolic) acidosis.26In the Henderson-Hasselbalch equation (Equation 2), the pH is equal to a constant, pK, plus the logarithm of the base/acid ratio. 1,17,23 If we substitute [ 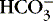 ] for the base and dissolved CO 2 for the acid, 8,23 multiplying CO 2 by its solubility coefficient (0.03 mEq/L/mm Hg), the equation becomes the following:
] for the base and dissolved CO 2 for the acid, 8,23 multiplying CO 2 by its solubility coefficient (0.03 mEq/L/mm Hg), the equation becomes the following:
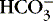 ] for the base and dissolved CO 2 for the acid, 8,23 multiplying CO 2 by its solubility coefficient (0.03 mEq/L/mm Hg), the equation becomes the following:
] for the base and dissolved CO 2 for the acid, 8,23 multiplying CO 2 by its solubility coefficient (0.03 mEq/L/mm Hg), the equation becomes the following:(4)
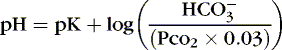
The value of pK is 6.1; normal [  ] is 24 mEq/L, and normal Pa co2 is 40 mm Hg.
] is 24 mEq/L, and normal Pa co2 is 40 mm Hg.
 ] is 24 mEq/L, and normal Pa co2 is 40 mm Hg.
] is 24 mEq/L, and normal Pa co2 is 40 mm Hg.Substituting, we obtain the following:
(5)
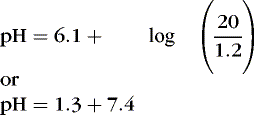
1. Hypoventilation of sufficient degree that Pa co2 is doubled from 40 to 80 (respiratory acidosis) results in a ratio of 24:2.4, or 10. The logarithm of 10 is 1, and the pH would be 6.1 + 1, or 7.1.
2. If a metabolic acidosis reduced [ 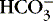 ] from 24 mEq/L to 12 mEq/L, the ratio would be 12:1.2 or 10:1, and the pH would be 7.1.
] from 24 mEq/L to 12 mEq/L, the ratio would be 12:1.2 or 10:1, and the pH would be 7.1.
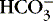 ] from 24 mEq/L to 12 mEq/L, the ratio would be 12:1.2 or 10:1, and the pH would be 7.1.
] from 24 mEq/L to 12 mEq/L, the ratio would be 12:1.2 or 10:1, and the pH would be 7.1.MIXED CONTRIBUTIONS
Thus far, these derangements (Figure 8-1) have been discussed as if they happened in isolation, but combined respiratory and nonrespiratory problems often occur, depending on pathologic processes in the body. Besides the four single acid-base derangements, there are combined acid-base derangements: (1) respiratory acidosis and metabolic acidosis, (2) respiratory acidosis and metabolic alkalosis, (3) respiratory alkalosis and metabolic acidosis, and (4) respiratory alkalosis and metabolic alkalosis. The combined acidoses or combined alkaloses have a cumulative effect on the pH, whereas an acidosis and alkalosis combination tends to negate the effects of each on the pH value. 16,17,21,31
COMPENSATION
Acid-base homeostasis maintains pH near the normal range. Thus if either the respiratory or nonrespiratory acid-base system is “deranged,” the other system will become “unbalanced” in the opposite direction to counterbalance the primary process. The body attempts to maintain equilibrium by balancing a pathologic process with a physiologic process or predictable buffering response. 3,17,28 For example, any respiratory process that leads to retention of carbon dioxide (respiratory acidosis) stimulates a nonrespiratory system, in this case the renal system, to return pH toward normal. This occurs by renal retention of bicarbonate and corresponding excretion of hydrogen ions. Given sufficient time, this may increase blood bicarbonate by as much as 3 to 4 mEq/L for each 10 mm Hg increase in carbon dioxide. Thus a neonate with a chronically increased Pa co2 and a compensatory rise in bicarbonate may attain a near-normal pH. 17
Metabolic compensations to respiratory processes can go to remarkable extremes, but respiratory compensations to metabolic processes are limited. Hyperventilation cannot lower the Pa co2 much below 10 mm Hg in compensation for a metabolic acidosis. Similarly, hypoventilation is limited in compensation for a metabolic alkalosis by the onset of hypoxemia. 28 Hypoxemia stimulates respiratory drive, overriding compensatory hypoventilation, limiting correction of alkalemia. 17
CORRECTION
Correction of an acid-base disturbance occurs when the health care provider detects the pathophysiologic process and directs therapy at the primary pathologic process, rather than counterbalancing it with a second pathologic process.
For example, if a respiratory acidosis is present, the clinician assesses the patient to discover the cause of the carbon dioxide retention and directs therapy at improving the ventilatory capacity of the lung, rather than attempting to increase the retention of bicarbonate.
Oxygenation
The remaining components of the blood gas analysis are the P o2, hemoglobin, and oxygen saturation. 21 Oxygenation is related to but also distinct from ventilation. 3The two main factors contributing to oxygenation at the tissue level are oxygen delivery and oxygen consumption. Oxygen delivery is the product of the cardiac output and the oxygen carrying capacity of blood, whereas oxygen consumption is determined by the metabolic needs of the body’s tissues. Tissue hypoxia may be caused by many different factors that derange the balance between oxygen delivery and tissue needs. Inability of the lung to oxygenate the blood would decrease oxygen delivery because of arterial hypoxemia. Another cause of tissue hypoxia is interference with blood flow, as in heart failure. The Pa o2 may be normal, but because of heart (pump) failure, oxygenated blood is not delivered in sufficient quantity. Treatment should be directed toward improving delivery by the pump (see Chapter 24). A third cause of tissue hypoxia is decreased blood oxygen–carrying capacity as with anemia. In this instance, the heart and lungs work adequately, so Pa o2 is normal but hemoglobin is insufficient to provide adequate oxygen. Finally, tissue hypoxia may result from an abnormally high affinity of oxygen for hemoglobin. Because oxyhemoglobin affinity is increased, oxygen will not dissociate from hemoglobin unless the venous, and therefore tissue, P o2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree